
3-(2-ETHOXYCARBONYL-ACETYL)-PIPERIDINE-1-CARBOXYLIC ACID BENZYL ESTER synthesis
- Product Name:3-(2-ETHOXYCARBONYL-ACETYL)-PIPERIDINE-1-CARBOXYLIC ACID BENZYL ESTER
- CAS Number:672323-13-6
- Molecular formula:C18H23NO5
- Molecular Weight:333.38
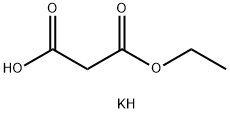
6148-64-7
403 suppliers
$5.00/10g
![1-[(Benzyloxy)carbonyl]-3-piperidinecarboxylic acid](/CAS/GIF/78190-11-1.gif)
78190-11-1
219 suppliers
$39.00/1g

672323-13-6
19 suppliers
$124.00/0.25/ G
Yield:672323-13-6 77%
Reaction Conditions:
with 1,1 ‘-carbonyldiimidazole;magnesium(II) chloride in acetonitrile;
Steps:
104.1 Step 1
To 1-((benzyloxy)carbonyl)piperidine-3-carboxylic acid (5.0 g, 18.99 mmol) in acetonitrile (60 mL) was added CDI (3.70 g, 22.79 mmol), in portions. After 1 h, potassium 3-ethoxy-3-oxopropanoate (3.23 g, 18.99 mmol) and magnesium chloride (1.808 g, 18.99 mmol) were added, and the resulting mixture was stirred overnight, concentrated, diluted with water, neutralized with citric acid and extracted with EtOAc (3X). The combined organic extracts were washed with brine (2X), dried over Na2SO4, filtered, concentrated and purified via normal phase chromatography (Combiflash Rf, 120 g silica column), eluting with 0-50% EtOAc in hexanes to afford benzyl 3-(3-ethoxy-3-oxopropanoyl)piperidine-1-carboxylate (4.86 g, 14.58 mmol, 77 % yield) as a colorless oil. LCMS: (ES, m/s) 334 [M+H]+. 1H NMR: (400 MHz, DMSO-d6) δ ppm 7.29-7.40 (m, 5H), 5.07 (d, J=1.5 Hz, 2H), 4.05-4.13 (m, 2H), 3.94-4.01 (m, 1H), 3.79 (br d, J=13.2 Hz, 1H), 3.67 (s, 2H), 2.82-3.11 (m, 2H), 2.61-2.70 (m, 1H), 1.94-2.02 (m, 1H), 1.60-1.69 (m, 1H), 1.33-1.51 (m, 2H), 1.12-1.23 (m, 3H).
References:
WO2022/152852,2022,A1 Location in patent:Page/Page column 187