2-(5-Oxo-6,7,8,9-Tetrahydro-5H-Benzo[7]Annulen-6-Yl)Acetic Acid synthesis
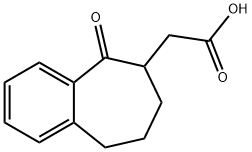
- Product Name:2-(5-Oxo-6,7,8,9-Tetrahydro-5H-Benzo[7]Annulen-6-Yl)Acetic Acid
- CAS Number:6742-32-1
- Molecular formula:C13H14O3
- Molecular Weight:218.25

25742-81-8
1 suppliers
inquiry
![2-(5-Oxo-6,7,8,9-Tetrahydro-5H-Benzo[7]Annulen-6-Yl)Acetic Acid](/CAS/20180808/GIF/6742-32-1.gif)
6742-32-1
18 suppliers
inquiry
Yield:6742-32-1 97%
Reaction Conditions:
Stage #1: ethyl 2-(5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulene-6-yl)acetatewith potassium hydroxide;ethanol;water monomer for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride;water monomer at 0; pH=~ 2.0;
Steps:
5
SYNTHETIC PREPARATION 5; Synthesis of 2-(5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-6-yl)acetic acidCompound of formula (Dc)The compound of formula (Db), ethyl 2-(5-oxo-6,7,8,9-tetrahydro-5/-/- benzo[7]annulen-6-yl)acetate, (6.6 g, 26.8 mmol) was dissolved in ethanol (EtOH) (30 ml_), then 10% potassium hydroxide (KOH) aqueous solution (37.5 ml_, 67 mmol) was added and the resulting mixture was refluxed for 2 h. After cooling to ambient temperature, the EtOH was removed by evaporation. The residue was extracted with EtOAc twice (15 mL x 2). The aqueous layer was then transferred into a flask and cooled with an ice-water bath, con. HCI was added dropwise to adjust pH to about 2.0. EtOAc (60 mL) was then added, the layers were separated, and the aqueous layer was extracted with EtOAc. The combined extracts were washed with brine. After being dried (MgSO4), filtered, and concentrated, the compound of formula (Dc), 2-(5-oxo- 6,7,8,9-tetrahydro-5H-benzo[7]annulen-6-yl)acetic acid, was obtained as an orange oil (5.7 g, 97%); 1H NMR (300 MHz, CDCI3) δ: 7.71-7.68 (m, 1 H), 7.41-7.20 (m, 3H), 3.37- 3.31 (m, 1 H), 3.12-2.91 (m, 3H), 2.57-2.49 (m, 1 H), 2.15-1.90 (m, 2H), 1.75-1.62 (m, 2H); LC-MS: purity: 100%; MS (m/e) : 219 (MH+).
References:
WO2008/83353,2008,A1 Location in patent:Page/Page column 125

130922-07-5
0 suppliers
inquiry
![2-(5-Oxo-6,7,8,9-Tetrahydro-5H-Benzo[7]Annulen-6-Yl)Acetic Acid](/CAS/20180808/GIF/6742-32-1.gif)
6742-32-1
18 suppliers
inquiry
![2H-Benzo[6,7]cyclohepta[1,2-b]furan-2,3(4H)-dione, 5,6-dihydro-](/CAS/20210305/GIF/7248-68-2.gif)
7248-68-2
0 suppliers
inquiry
![2-(5-Oxo-6,7,8,9-Tetrahydro-5H-Benzo[7]Annulen-6-Yl)Acetic Acid](/CAS/20180808/GIF/6742-32-1.gif)
6742-32-1
18 suppliers
inquiry
![methyl 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulene-6-carboxylate](/CAS/20200611/GIF/74821-55-9.gif)
74821-55-9
7 suppliers
inquiry
![2-(5-Oxo-6,7,8,9-Tetrahydro-5H-Benzo[7]Annulen-6-Yl)Acetic Acid](/CAS/20180808/GIF/6742-32-1.gif)
6742-32-1
18 suppliers
inquiry