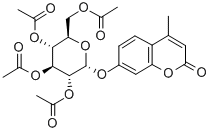
4-Methylumbelliferyl2,3,4,6-tetra-O-acetyl-a-D-glucopyranoside synthesis
- Product Name:4-Methylumbelliferyl2,3,4,6-tetra-O-acetyl-a-D-glucopyranoside
- CAS Number:67945-53-3
- Molecular formula:C24H26O12
- Molecular Weight:506.46
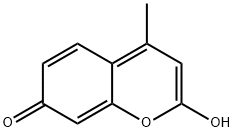
79566-13-5
9 suppliers
inquiry
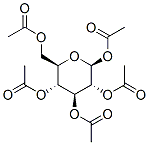
604-69-3
398 suppliers
$10.00/25g
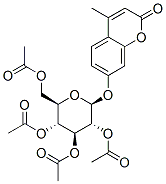
67909-25-5
15 suppliers
$130.00/100mg

67945-53-3
30 suppliers
$52.50/50mg
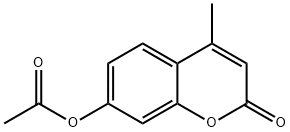
2747-05-9
174 suppliers
$9.00/1g
Yield:67909-25-5 45% ,67945-53-3 7% ,2747-05-9 16%
Reaction Conditions:
with dmap;boron trifluoride diethyl etherate in 1,2-dichloro-ethane at 60; for 5 h;Inert atmosphere;stereoselective reaction;Helferich Method;
Steps:
General Procedure for the Glycosylation Step
General procedure: To a mixture of 4-MU (1.0-6.0 mmol) and glycosyl acetate (1.0-4.0 mmol) under an argon atmosphere, dry solvent was added successively, followed by the corresponding molar equivalents of base and Lewis acid. The mixture was stirred for a set amount of time at a certain temperature (as shown in Tables 1-3). Then, an equal volume of CH2Cl2 was added to dilute the reaction mixture, and the reaction was quenched with saturated aqueous NaHCO3. The organic phase was washed with diluted aqueous NaOH (1 M) until the aqueous phase was a light brownish-yellow or almost colorless, then washed successively with distilled water, saturated aqueous NaCl, dried with anhydrous Na2SO4, and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography (silica gel, 200-300 mesh, PE/EtOAc, 5/2), then the desired product was crystallized from anhydrous ethyl ether and dried, or the crude product was purified by several recrystallizations from ethanol.
References:
Wei, Xianhu;Ma, Yanxia;Wu, Qingping;Zhang, Jumei;Cai, Zhihe;Lu, Mianfei;Ferro, Vito [Molecules,2015,vol. 20,# 12,p. 21681 - 21699]
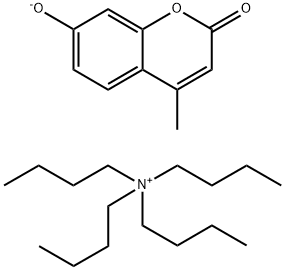
84380-11-0
0 suppliers
inquiry
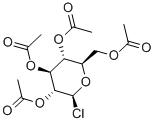
4451-36-9
46 suppliers
$45.00/50mg

67945-53-3
30 suppliers
$52.50/50mg

79566-13-5
9 suppliers
inquiry

67945-53-3
30 suppliers
$52.50/50mg