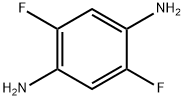
2,5-Difluorophenylene-1,4-diamine, 1,4-Diamino-2,5-difluorobenzene synthesis
- Product Name:2,5-Difluorophenylene-1,4-diamine, 1,4-Diamino-2,5-difluorobenzene
- CAS Number:698-52-2
- Molecular formula:C6H6F2N2
- Molecular Weight:144.12
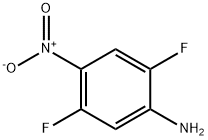
1542-36-5
44 suppliers
$95.00/100mg

698-52-2
25 suppliers
$198.00/50mg
Yield: 88%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; for 5 h;Inert atmosphere;
Steps:
3
To a solution of compound 3.4 (2.50 g, 14.36 mmol, 1 equiv) in MeOH (30 mL) was added Pd/C (600 mg, 10% purity) under N2. The suspension was degassed under vacuum and purged with H2 several times. The mixture was stirred under H2 (15 psi) at 20 °C for 5 hr. LC- MS showed compound 3.4 was consumed, and the main product was compound 3.5 (r.t. = 0.114 min, [M+H]+ = 144.7). The reaction mixture was filtered through a celite pad, and the filtrate was concentrated under reduced pressure to give compound 3.5 (1.82 g, 12.63 mmol. 88.0% yield) as a black-brown solid. NMR (400 MHz, DMSO-de): d 6.48 (t, J = 10.4 Hz, 2H), 4.45 (br s, 4H).
References:
FOG PHARMACEUTICALS, INC.;MCGEE, John, Hanney;THOMSON, Ty, Matthew;WAHL, Sebastian, Christof, Theodor;VERDINE, Gregory, L.;REZAEI ARAGHI, Raheleh;ZHANG, Yue-Mei;MULVIHILL, Mark Joseph WO2020/41270, 2020, A1 Location in patent:Paragraph 0352; 0356
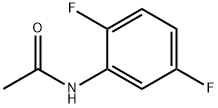
398-90-3
56 suppliers
$75.00/1g

698-52-2
25 suppliers
$198.00/50mg
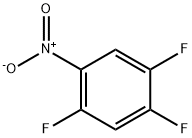
2105-61-5
211 suppliers
$6.00/5g

698-52-2
25 suppliers
$198.00/50mg
404-01-3
47 suppliers
$31.00/1 g

698-52-2
25 suppliers
$198.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

698-52-2
25 suppliers
$198.00/50mg