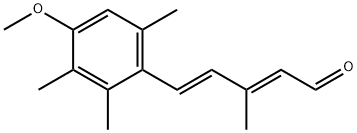
(2E,4E)-5-(4-methoxy-2,3,6-trimethylphenyl)-3-methylpenta-2,4-dienal synthesis
- Product Name:(2E,4E)-5-(4-methoxy-2,3,6-trimethylphenyl)-3-methylpenta-2,4-dienal
- CAS Number:69877-38-9
- Molecular formula:C16H20O2
- Molecular Weight:244.33
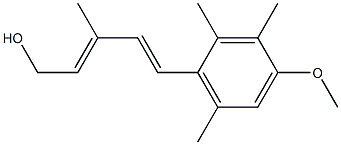
63650-98-6

69877-38-9
GENERAL METHOD: Sodium carbonate (4.77 g, 45 mmol) and activated manganese dioxide powder (3.91 g, 45 mmol) were added to a vigorously stirred solution of alcohols 27-31 (2.2 mmol) in dichloromethane (45 mL). The resulting suspension was stirred at room temperature until the reaction was complete (monitored by thin layer chromatography) and subsequently filtered through a diatomaceous earth pad. The filtrate was evaporated to dryness to obtain the residue. The residue was purified by fast column chromatography to afford allyl aldehydes 32-36 (only the 2E,4E-isomer was plotted).

63650-98-6
0 suppliers
inquiry

69877-38-9
41 suppliers
$156.00/100mg
Yield:69877-38-9 90%
Reaction Conditions:
with manganese(IV) oxide;sodium carbonate in dichloromethane at 25; for 1 h;
Steps:
4.1.5. General procedure for the MnO2-mediated oxidations of allylic alcohols
General procedure: To a vigorously stirred solution of alcohols 27-31 (2.2 mmol) in CH2Cl2 (45 mL), Na2CO3 (4.77 g, 45 mmol) and activated MnO2 powder (3.91 g, 45 mmol) were added. The resulting suspension was stirred at ambient temperature to complete the reaction (monitored by TLC) and then passed through a celite pad. The filtrate was evaporated to dryness to leave a residue. Allylic aldehydes 32-36 (only the 2E,4E-isomer is drawn) were obtained from this residue through FCC purification.
References:
Magoulas, George E.;Bariamis, Stavros E.;Athanassopoulos, Constantinos M.;Haskopoulos, Anastasios;Dedes, Petros G.;Krokidis, Marios G.;Karamanos, Nikos K.;Kletsas, Dimitris;Papaioannou, Dionissios;Maroulis, George [European Journal of Medicinal Chemistry,2011,vol. 46,# 2,p. 721 - 737] Location in patent:experimental part

478496-90-1
0 suppliers
inquiry

69877-39-0
0 suppliers
inquiry

69877-38-9
41 suppliers
$156.00/100mg
5469-19-2
84 suppliers
$26.39/25g

69877-38-9
41 suppliers
$156.00/100mg

154152-57-5
1 suppliers
inquiry

69877-38-9
41 suppliers
$156.00/100mg
20469-61-8
120 suppliers
$19.00/5g

69877-38-9
41 suppliers
$156.00/100mg