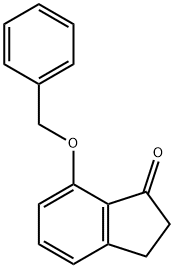
7-(benzyloxy)-2,3-dihydroinden-1-one synthesis
- Product Name:7-(benzyloxy)-2,3-dihydroinden-1-one
- CAS Number:125494-84-0
- Molecular formula:C16H14O2
- Molecular Weight:238.28
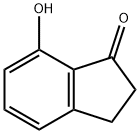
6968-35-0
112 suppliers
$17.00/250mg

100-39-0
424 suppliers
$10.00/10g

125494-84-0
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in acetone;Reflux;
Steps:
13.2
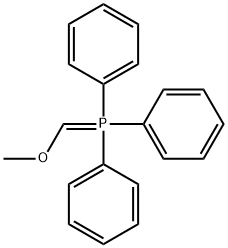
Step 2: A mixture of S13-1 (10.36 g, 70 mmol), benzyl bromide (12 g, 70 mmol) and potassium carbonate (10.63 g, 77 mmol) in acetone (300 mL) was refluxed overnight. Then the reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated and the residue was purified by column chromatography to afford S13-2. ‘H NMR (300 MHz, CDC13) ? 2.65 - 2.69 (m, 2H), 3.05 - 3.09 (m, 2H), 5.26 (s, 2H), 6.73 - 6.76 (d, J= 8.4 Hz, 1H), 6.96 -6.98 (d, J= 7.5 Hz, 1H), 7.24 -7.46 (m, 6H); LC-MS: (M+H) 239.
References:
WO2015/95261,2015,A1 Location in patent:Page/Page column 67
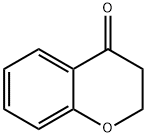
491-37-2
289 suppliers
$6.00/1g

125494-84-0
7 suppliers
inquiry