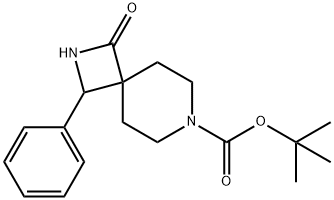
7-Boc-1-oxo-3-phenyl-2,7-diaza-spiro[3.5]nonane synthesis
- Product Name:7-Boc-1-oxo-3-phenyl-2,7-diaza-spiro[3.5]nonane
- CAS Number:1013033-83-4
- Molecular formula:C18H24N2O3
- Molecular Weight:316.39

100-52-7
976 suppliers
$20.37/250g
142851-03-4
286 suppliers
$5.00/1g
![7-Boc-1-oxo-3-phenyl-2,7-diaza-spiro[3.5]nonane](/CAS/GIF/1013033-83-4.gif)
1013033-83-4
16 suppliers
inquiry
Yield:1013033-83-4 67%
Reaction Conditions:
Stage #1: benzaldehydewith lithium hexamethyldisilazane in tetrahydrofuran at -30;
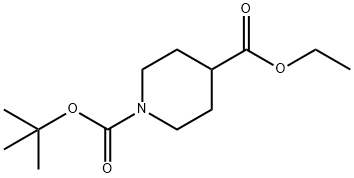
Stage #2: 1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylatewith lithium diisopropyl amide in tetrahydrofuran at -78 - 0; for 3 h;
Stage #3: with water in tetrahydrofuran at 0;Product distribution / selectivity;
Steps:
1
A first solution of LiHMDS (2.8 mole) in THF (1.0 L) was cooled to -30° C., and to the resultant mixture was added benzaldehyde (300 g, 2.8 mole) via dropwise addition with stirring. In a separate flask, a second solution of LDA (2.8 mole in THF) was cooled to -78° C. and a solution of BOC-ethylisonipecotate (687 g, 2.8 mole) in THF (500 mL) was added dropwise. The first solution was then added dropwise to the second solution with stirring at a rate such that the reaction temperature was maintained below 0° C. throughout the addition process. The resultant reaction was then allowed to stir for about 3 hours at 0° C., after which time the reaction was carefully quenched using of water (1 L). The resultant solution was extracted with ethyl acetate (5 L) and transferred to a separatory funnel. The organic layer was collected, dried using magnesium sulfate and concentrated in vacuo to provide a crude product which was recrystallized from 1.5 L of Hexane:ethyl acetate (2:1) provide compound IA-1 as a solid (67%). 1H NMR (300 MHz, DMSO) δ 7.4 (d, 2H), 7.3 (br, 3H), 4.6 (s, 1H), 3.5 (br, 2H), 3.2 (br, 1H), 3.0 (br, 1H), 1.9 (br, 2H), 1.3 (s, 9H), 1.2 (br, 1H), 1.0 (br, 1H)
References:
US2008/76750,2008,A1 Location in patent:Page/Page column 46; 50
![[1-PHENYL-METHYLIDENE]-TRIMETHYLSILANYL-AMINE](/CAS/GIF/17599-61-0.gif)
17599-61-0
17 suppliers
$132.00/5g

142851-03-4
286 suppliers
$5.00/1g
![7-Boc-1-oxo-3-phenyl-2,7-diaza-spiro[3.5]nonane](/CAS/GIF/1013033-83-4.gif)
1013033-83-4
16 suppliers
inquiry