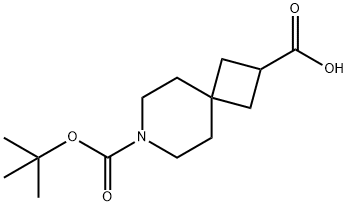
7-Azaspiro[3.5]nonane-2,7-dicarboxylic acid, 7-(1,1-dimethylethyl) ester synthesis
- Product Name:7-Azaspiro[3.5]nonane-2,7-dicarboxylic acid, 7-(1,1-dimethylethyl) ester
- CAS Number:873924-12-0
- Molecular formula:C14H23NO4
- Molecular Weight:269.34
![2-Cyano-7-azaspiro[3.5]nonane-7-carboxylic acid tert-butyl ester](/CAS/GIF/203662-66-2.gif)
203662-66-2
![7-Azaspiro[3.5]nonane-2,7-dicarboxylic acid, 7-(1,1-dimethylethyl) ester](/CAS/GIF/873924-12-0.gif)
873924-12-0
The general procedure for the synthesis of 7-(tert-butoxycarbonyl)-7-azaspiro[3.5]nonane-2-carboxylic acid using tert-butyl 2-cyano-7-azaspiro[3.5]nonane-7-carboxylate as a starting material was as follows: tert-butyl 2-cyano-7-azaspiro[3.5]nonane-7-carboxylate (1.5 g, 5.99 mmol) was dissolved in ethanol (40 mL) and water (40 mL) in a solvent mixture of ethanol (40 mL) and water (40 mL). Lithium hydroxide (880 mg, 21 mmol) was added to this solution and the reaction mixture was subsequently heated to reflux and held for 4 hours. After completion of the reaction, the mixture was cooled to room temperature. Ethanol was removed by rotary evaporator and the remaining aqueous phase was adjusted to pH 1-2 with 6N hydrochloric acid. next, the aqueous phase was extracted with ether. The organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated to give 7-(tert-butoxycarbonyl)-7-azaspiro[3.5]nonane-2-carboxylic acid (1.6 g, 99% yield) as a white solid. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 3.34-3.41 (m, 2H), 3.27-3.34 (m, 2H), 3.10-3.21 (m, 1H), 2.12 (d, J = 8.9 Hz, 4H), 1.58-1.63 (m, 2H), 1.52-1.58 (m, 2H), 1.47 ( s, 9H). Mass spectral analysis showed m/z 214 ([MH - t-Bu]+), 292 ([MNa]+).
![2-Cyano-7-azaspiro[3.5]nonane-7-carboxylic acid tert-butyl ester](/CAS/GIF/203662-66-2.gif)
203662-66-2
83 suppliers
inquiry
![7-Azaspiro[3.5]nonane-2,7-dicarboxylic acid, 7-(1,1-dimethylethyl) ester](/CAS/GIF/873924-12-0.gif)
873924-12-0
88 suppliers
$108.00/1g
Yield: 99%
Reaction Conditions:
Stage #1:2-cyano-7-azaspiro[3.5]nonane-7-carboxylic acid tert-butyl ester with water;lithium hydroxide in ethanol for 4 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=1 - 2
Steps:
35
Synthesis of 7-(tert-butoxycarbonyl)-7-azaspiro[3.5]nonane-2-carboxylic acid; tert-Butyl 2-cyano-7-azaspiro[3.5]nonane-7-carboxylate (1.5 g, 5.99 mmol) was dissolved in ethanol (40 mL) and water (40 mL). Lithium hydroxide (880 mg, 21 mmol) was added and the mixture was heated to reflux for 4 hours. The reaction mixture was cooled to room temperature. The ethanol was evaporated and the aqueous layer was acidified (pH 1-2) with 6N HCl. The aqueous layer was extracted with diethyl ether. The organic layer was washed with brine, dried (MgSO4), filtered and concentrated to give the title compound as a white solid (1.6 g, 99%). 1H NMR (400 MHz, CDCl3) δ ppm 3.34-3.41 (m, 2H), 3.27-3.34 (m, 2H), 3.10-3.21 (m, 1H), 2.12 (d, J=8.9 Hz, 4H), 1.58-1.63 (m, 2H), 1.52-1.58 (m, 2H), 1.47 (s, 9H). m/z 214 (MH+ minus t-Bu), 292 (MNa+).
References:
Pfizer Inc. US2010/113465, 2010, A1 Location in patent:Page/Page column 32