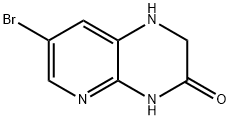
7-BroMo-1,2-dihydropyrido[2,3-b]pyrazin-3(4H)-one synthesis
- Product Name:7-BroMo-1,2-dihydropyrido[2,3-b]pyrazin-3(4H)-one
- CAS Number:957198-15-1
- Molecular formula:C7H6BrN3O
- Molecular Weight:228.05
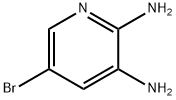
38875-53-5
348 suppliers
$6.00/1g
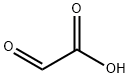
298-12-4
434 suppliers
$5.00/25g
![7-BroMo-1,2-dihydropyrido[2,3-b]pyrazin-3(4H)-one](/CAS/20150408/GIF/957198-15-1.gif)
957198-15-1
17 suppliers
$130.00/50mg
Yield:957198-15-1 96%
Reaction Conditions:
Stage #1: 5-bromo-pyridine-2,3-diamine;Glyoxilic acid in water at 20; for 48 h;
Stage #2: with methanol;sodium tetrahydroborate at 0 - 20; for 2 h;
Stage #3: with hydrogenchloride in water at 50; for 2 h;
Steps:
6.1 Step 1: 7-Bromo- 1 ,4-dihydropyrido [2, 3-blpyrazin-3(2H)-one (B39-2)
A mixture of 5-bromopyridine-2,3-diamine (2 g, 10.6 mmol) and 50 % 2-oxoacetic acid (2 g, 13.8 mmol) in 50 mL of H20 was stirred at room temperature for 48 h. The reaction was filtered and the filter cake was washed by water. The residue was dissolved in 50 mL of methanol and added NaBH4 (1 g, 26.0 mmol) in portions at 0 °C. The mixture was stirred at room temperature for 2 h. Then the reaction was added 10 mL of 6N HC1. After stirring at room temperature for an hour, then the reaction was warmed to 50 °C for 2 h. The resulting reaction was evaporated and adjusted pH to 8 by saturated aqueous NaHCO3. Then the mixture was filtered to give a gray solid (2.3 g, 96 %). ‘H NMR (400 MHz, DMSO-d6) ? 7.64 (s, 1H), 7.01-6.98 (m, 2H), 3.93 (s, 2H).
References:
WO2018/237370,2018,A1 Location in patent:Paragraph 00153; 00154; 00155