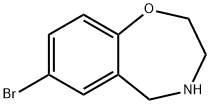
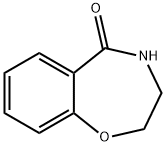
7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine synthesis
- Product Name:7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine
- CAS Number:740842-71-1
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
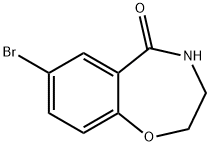
5755-05-5
31 suppliers
inquiry
![7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine](/CAS/20180703/GIF/740842-71-1.gif)
740842-71-1
8 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 7-bromo-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-onewith borane-THF in tetrahydrofuran at 20 - 65;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water; for 1 h;Heating / reflux;
Stage #3: with sodium hydroxide in water;
Steps:
72
Preparation 72: 7-Bromo-2,3,4,5-tetrahydro-1,4-benzoxazepine. A solution of 7-bromo-3,4-dihydro-1 ,4-benzoxazepin-5(2H)-one (Preparation 71 ) (3.28 g, 13.55 mmol) in tetrahydrofuran (THF) (30ml) was stirred under argon. Borane tetrahydrofuran complex (81 ml, 81 mmol) was added slowly and the solution stirred at RT for 1.5 hrs. The solution was heated at 650C overnight. The reaction was cooled and MeOH (20ml) added dropwise until fizzing ceased. Then 2M HCI (100ml) was added and the reaction heated at reflux for 1 hour before being cooling and solvent removal by evaporation. The reaction mixture was partitioned between EtOAc/Water and separated. The aqueous layer was basified with 2M NaOH, extracted with EtOAc, dried over magnesium sulphate and evaporated to give a colourless oil (1g). Precipitated solid from the initial EtOAc layer was filtered and dried. This solid was dissolved in EtOAc/ 2M NaOH, separated and the organic layer dried over magnesium sulphate and evaporated to give a colourless oil (1.6g). The 2 products were combined to give the title compound (2.6g) as a colourless oil. MS (ES): C9H1081BrNO requires 229; found 230 [M+H]+.
References:
WO2009/80725,2009,A1 Location in patent:Page/Page column 68
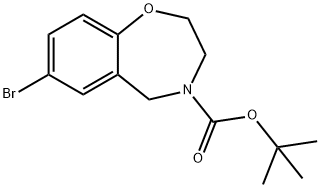
740842-73-3
27 suppliers
$100.00/1g
![7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine](/CAS/20180703/GIF/740842-71-1.gif)
740842-71-1
8 suppliers
inquiry
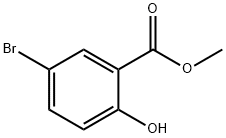
4068-76-2
182 suppliers
$8.00/1g
![7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine](/CAS/20180703/GIF/740842-71-1.gif)
740842-71-1
8 suppliers
inquiry

1167416-56-9
1 suppliers
inquiry
![7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine](/CAS/20180703/GIF/740842-71-1.gif)
740842-71-1
8 suppliers
inquiry
703-51-5
55 suppliers
$120.00/250mg
![7-bromo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine](/CAS/20180703/GIF/740842-71-1.gif)
740842-71-1
8 suppliers
inquiry
![2,3,4,5-Tetrahydrobenzo[f][1,4]oxazepine](/CAS/GIF/17775-01-8.gif)
17775-01-8
61 suppliers
$45.00/25mg