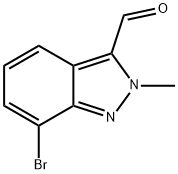
7-Bromo-2-methyl-2H-indazole-3-carbaldehyde synthesis
- Product Name:7-Bromo-2-methyl-2H-indazole-3-carbaldehyde
- CAS Number:845751-70-4
- Molecular formula:C9H7BrN2O
- Molecular Weight:239.07
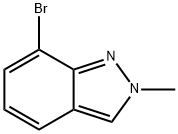
701910-14-7
104 suppliers
$50.00/50mg

845751-70-4
21 suppliers
$119.00/100mg
Yield:845751-70-4 62%
Reaction Conditions:
Stage #1: 7-bromo-2-methyl-2H-indazolewith lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78 - 0; for 0.5 h;
Stage #2: with N,N-dimethyl-formamide at -78 - 20; for 19 h;Product distribution / selectivity;
Steps:
12.1; 30.1
Example 12 7- (2,4-Dichloro-phenyl)-3-ethynyl-2-methyl-2H-indazole step 1 2-methyl-7-bromoindazole (6: R = Me; 5.31 g, 25.16 mmol) in THF (100 mL) was cooled to-78° C under an Ar atmosphere. A 2 M solution of LDA in heptane/THF/ethylbenzene (20 mL, 40 mmol) was added slowly. The mixture was then stirred at-78 °C for 10 minutes and 0° C for 20 minutes. The solution was re-cooled TO-78° C, DMF (6 mL, 77. 48 mmol) was added slowly via syringe. The mixture was stirred and allowed to warm to room temperature for 19 hours. The reaction was partitioned between EtOAc and NH4C1 solution. The aqueous layer was extracted with EtOAc two times. The combined organic layers were dried over MgS04 and concentrated to almost dryness. The resulting yellow solids (3.83 g) were collected by filtration and washed with 10% EtOAc in hexanes. The filtrate was concentrated and the residue purified by SI02 chromatography and eluted with a EtOAc/hexane (5--*30 %) over 30 minutes to provide an additional crop of 17a (0.46 g, total yield 62%) along with the recovered starting material (0.768 g, 14%).; Example 30 [7-(2, 4-Dichloro-phenyl)-2-methyl-2H-indazol-3-ylmethyl]-carbamic acid methyl ester; hydrochloride CHO step 1 ~ NN-Me Br Br Ar 6 : R = Me 80 step 3 = : R =CHO 81 82 Ar 2, 4-dichlorophenyl step2 ~step 1 To a solution of 7-bromo-2-methyl-indazole (6, R = Me, 3.20 g, 15.16 mmol) and dry THF (50 mL) which was cooled to-78° C and maintained under an N2 atmosphere was added dropwise LDA (12.0 mL, 22.74 mmol, 2. 0M solution in heptane/THF/ethylbenzene). After the addition was completed the reaction mixture was stirred for 10 min and warmed to 0° C for 20 min. The dark red solution was cooled to- 78° C and DMF (3.0 mL, 45.48 mmol) was added dropwise. The solution was allowed to warm to RT and stirred overnight. The reaction was quenched by the addition of saturated NH4C1 (50 mL) and the resulting solution was twice extracted with EtOAc. The combined extracts were dried (MGS04), filtered and evaporated. The crude product was purified by flash chromatography on SI02 (0 to 40% EtOAc/heptane in a linear gradient over 20 min) to afford 0.890 g of solid which was a mixture of starting material and the desired product. The reaction was rechromatographed on Si02 (0 to 20% EtOAc/heptane in a linear gradient over 20 min) to afford pure 80 (0.470 g) as solid. A second fraction contained 1.22 g of a mixture of starting material and the desired product.
References:
WO2005/16892,2005,A1 Location in patent:Page/Page column 80; 96
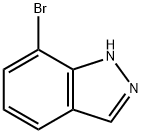
53857-58-2
199 suppliers
$7.00/250mg

845751-70-4
21 suppliers
$119.00/100mg