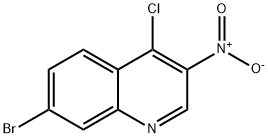
7-bromo-4-chloro-3-nitroquinoline synthesis
- Product Name:7-bromo-4-chloro-3-nitroquinoline
- CAS Number:723280-98-6
- Molecular formula:C9H4BrClN2O2
- Molecular Weight:287.5
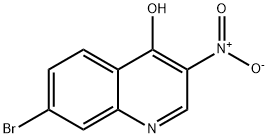
723280-94-2

723280-98-6
General procedure for the synthesis of 7-bromo-4-chloro-3-nitroquinolin-4-ol from 7-bromo-3-nitroquinolin-4-ol: 7-bromo-3-nitroquinolin-4-ol (3.8 g, 14.12 mmol) was suspended in phosphorous trichloride (20 mL) and anhydrous N,N-dimethylformamide (1 mL) was added. The reaction mixture was heated to 120 °C and maintained for 3 h under nitrogen protection. After completion of the reaction, it was cooled to room temperature. The precipitate was collected by filtration, washed with water and subsequently partitioned between dichloromethane (60 mL) and saturated aqueous sodium carbonate solution. The organic layer was separated, washed with saturated saline, dried over anhydrous sodium sulfate, filtered and concentrated to give 7-bromo-4-chloro-3-nitroquinoline (3.3 g, 11.5 mmol, 81%) as a beige solid. Mass spectrum (electrospray ionization, m/z): [M + H]+ = 287.1 / 288.9.

723280-94-2
29 suppliers
inquiry

723280-98-6
61 suppliers
$43.00/100mg
Yield:723280-98-6 92%
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-formamide at 85;Inert atmosphere;
Steps:
1.2; 5.2
Step 2: To a solution of compound 5-2 (25.0 g, 93.75 mmol) was in POCh (70 mL) was added anhydrous DMF (5 mL). The reaction mixture was heated to 85 °C under nitrogen overnight. The reaction mixture was cooled to room temperature. The precipitate was collected by fdtration, washed with water and dried in high vacuum to give compound 5-3 (24.0 g, yield: 92%) as a yellow solid.
References:
WO2020/163118,2020,A1 Location in patent:Paragraph 00154-00155; 00157-00158; 00182-00184
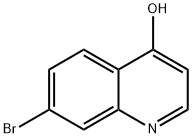
82121-06-0
138 suppliers
$49.00/1g

723280-98-6
61 suppliers
$43.00/100mg