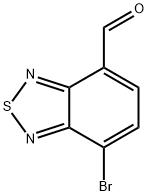
7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde synthesis
- Product Name:7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde
- CAS Number:1071224-34-4
- Molecular formula:C7H3BrN2OS
- Molecular Weight:243.08
![4-BroMo-7-(dibroMoMethyl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/1239277-96-3.gif)
1239277-96-3
![7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/CAS/20180629/GIF/1071224-34-4.gif)
1071224-34-4
General procedure for the synthesis of 7-bromo-4-formylbenzo[C][1,2,5]thiadiazole from 4-bromo-7-(dibromomethyl)benzo[C][1,2,5]thiadiazole: aqueous silver nitrate (285 mg, 1.68 mmol, dissolved in 1.7 mL of water) was added to a stirred solution of compound A (260 mg, 0.67 mmol) in acetonitrile (8 mL) , followed by heating and refluxing for 2 hours. Upon completion of the reaction, it was cooled to room temperature and the reaction mixture was filtered to remove the resulting AgBr precipitate. The filtrate was extracted with dichloromethane, the organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate and filtered. The solvent was removed by rotary evaporation to afford compound B (7-bromo-2,1,3-benzothiadiazole-4-carbaldehyde) as a white solid (147 mg, 92% yield). The physical and spectral data of compound B were as follows: melting point 185-186 °C; IR (KBr) ν 3078, 3021, 2835, 2728, 1702, 1526, 1268, 1102, 937, 879 cm?1; 1H NMR (CDCl3, 400 MHz) δ 10.71 (s, 1H), 8.09-8.03 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 188.0, 153.8, 152.1, 131.9, 131.5, 126.7, 121.7; HRMS (FAB+) m/z Calculated value C7H379BrN2OS [M]+ 241.9149, Measured value 241.9149; Calculated value C7H381BrN2OS [M]+ 243.9129, measured value 243.9137.
![4-BroMo-7-(dibroMoMethyl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/1239277-96-3.gif)
1239277-96-3
20 suppliers
$45.00/10mg
![7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/CAS/20180629/GIF/1071224-34-4.gif)
1071224-34-4
109 suppliers
$36.97/25G
Yield:1071224-34-4 80%
Reaction Conditions:
with formic acid at 120; for 24 h;
Steps:
1 Synthesis of Br-CHO:
Intermediate 3 (30.74 g, 79.45 mmol, 1.0 equivalent) and formic acid (100 mL) were sequentially added to a dry three-necked flask equipped with magnetic stirring, placed in an oil bath, and heated to 120°C.After 24 hours, the reaction was monitored by thin layer chromatography until the reaction was complete.The resulting mixture was cooled to room temperature, quenched by adding water and stirred for 1 hour.The resulting mixture was concentrated in vacuum, the residue was washed three times with water, and finally dried in a vacuum drying oven to obtain 15.45 g of brown solid compound with a yield of 80%
References:
CN111471043,2020,A Location in patent:Paragraph 0050; 0051; 0055
![4-BROMO-7-BROMOMETHYL-BENZO[1,2,5]THIADIAZOLE](/CAS/GIF/2255-78-9.gif)
2255-78-9
8 suppliers
inquiry
![7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/CAS/20180629/GIF/1071224-34-4.gif)
1071224-34-4
109 suppliers
$36.97/25G

1210936-24-5
0 suppliers
inquiry
![7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/CAS/20180629/GIF/1071224-34-4.gif)
1071224-34-4
109 suppliers
$36.97/25G
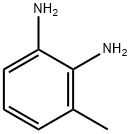
2687-25-4
285 suppliers
$16.00/1g
![7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/CAS/20180629/GIF/1071224-34-4.gif)
1071224-34-4
109 suppliers
$36.97/25G
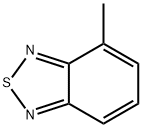
1457-92-7
83 suppliers
$31.00/250mg
![7-bromo-benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/CAS/20180629/GIF/1071224-34-4.gif)
1071224-34-4
109 suppliers
$36.97/25G