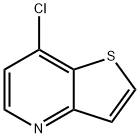
7-Chlorothieno[3,2-b]pyridine synthesis
- Product Name:7-Chlorothieno[3,2-b]pyridine
- CAS Number:69627-03-8
- Molecular formula:C7H4ClNS
- Molecular Weight:169.63
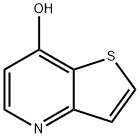
![THIENO[3,2-B]PYRIDIN-7-OL](/StructureFile/ChemBookStructure4/GIF/CB2324535.gif)
69627-02-7
![7-Chlorothieno[3,2-b]pyridine](/CAS/GIF/69627-03-8.gif)
69627-03-8
To a 250 mL round-bottomed flask were added 30 mL of methylene chloride and 20 mL of ethylene chloride, followed by N,N-dimethylformamide (1.8 mL, 23.28 mmol) and oxalyl chloride (2.9 mL, 33.86 mmol). Oxalyl chloride was slowly added dropwise to the mixture at 0 °C. Next, thieno[3,2-b]pyridin-7(4H)-one (1.6 g, 10.58 mmol) was added and the reaction mixture was heated to reflux for 6 hours. After completion of the reaction, the mixture was cooled to room temperature to afford 7-chlorothieno[3,2-b]pyridine (1.7 g, 90% yield) as a pale yellow solid. The product was characterized by 1H-NMR (400 MHz, CDCl3): δ 8.61 (d, J = 5.2 Hz, 1H), 7.81 (d, J = 5.2 Hz, 1H), 7.30 (d, J = 4.8 Hz, 1H).
107818-20-2
157 suppliers
$10.00/250mg
![7-Chlorothieno[3,2-b]pyridine](/CAS/GIF/69627-03-8.gif)
69627-03-8
172 suppliers
$6.00/100mg
Yield:69627-03-8 96%
Reaction Conditions:
Stage #1: thieno[3,2-b]pyridin-7-olwith trichlorophosphate at 60 - 100; for 3.5 h;
Stage #2: with ammonia;water monomer at 0;
Steps:
1
7-Chlorothieno[3,2-6]pyridine (6); To neat POCl3 (200 mL, 2146 mmol) at 6O0C in a 500 mL round-bottom flask was added thieno[3,2-&]pyridin-7-ol (1 eq., 200 g, 1323 mmol) in small portions over 1.5 h. The reaction mixture was heated at 6O0C for Ih and at 1000C for an additional hour. After cooling down to O0C, the reaction mixture was poured onto crushed ice (1 L) over a period of 30 min. After 15 min, NH4OH (1.5 L) was added to the mixture to form a grey precipitate that was collected by filtration, washed with water (50 mL) and air dried. The dry solid was suspended in EtOAc (1 L). The slurry was stirred at r.t. for 15 min, filtered and the filter was washed with EA (2x 100 mL). The organic phase was collected, dried over MgSO4 and concentrated. The residue was passed through a short silica gel pad (300 g, eluent - a gradient hexane/EtOAc, 8/2 to 5/5) and dried in the vacuum oven (350C) for 2h to afford 6 as a off-white solid (214.8 g, 1266 mmol, 96% yield). MS (m/z): 170.0 (M+H). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.64 (d, J = 5.1 Hz, IH), 8.25 (d, J = 5.5 Hz, IH), 7.66 (d, J = 5.5 Hz, IH), 7.54 (d, J = 5.1 Hz, IH).
References:
WO2009/26720,2009,A1 Location in patent:Page/Page column 93; 94
![THIENO[3,2-B]PYRIDIN-7-OL](/StructureFile/ChemBookStructure4/GIF/CB2324535.gif)
69627-02-7
19 suppliers
inquiry
![7-Chlorothieno[3,2-b]pyridine](/CAS/GIF/69627-03-8.gif)
69627-03-8
172 suppliers
$6.00/100mg
913377-45-4
11 suppliers
inquiry
![7-Chlorothieno[3,2-b]pyridine](/CAS/GIF/69627-03-8.gif)
69627-03-8
172 suppliers
$6.00/100mg
![Thieno[3,2-b]pyridine, 7-chloro-, 4-oxide](/CAS/20210305/GIF/104273-29-2.gif)
104273-29-2
0 suppliers
inquiry
![7-Chlorothieno[3,2-b]pyridine](/CAS/GIF/69627-03-8.gif)
69627-03-8
172 suppliers
$6.00/100mg
![THIENO[3,2-B]PYRIDIN-7-OL](/StructureFile/ChemBookStructure4/GIF/CB2324535.gif)
69627-02-7
19 suppliers
inquiry

79-37-8
490 suppliers
$17.67/10gm:
![7-Chlorothieno[3,2-b]pyridine](/CAS/GIF/69627-03-8.gif)
69627-03-8
172 suppliers
$6.00/100mg