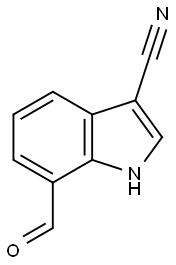
7-ForMyl-1H-indole-3-carbonitrile synthesis
- Product Name:7-ForMyl-1H-indole-3-carbonitrile
- CAS Number:1373137-75-7
- Molecular formula:C10H6N2O
- Molecular Weight:170.17
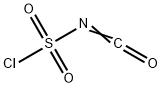
1189-71-5
341 suppliers
$18.00/5g
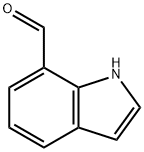
1074-88-0
213 suppliers
$8.00/250mg

1373137-75-7
4 suppliers
inquiry
Yield:1373137-75-7 91.3%
Reaction Conditions:
in acetonitrile at 5 - 25; for 2 h;
Steps:
1.1 (1) Synthesis of Compound 2 (7-formyl-1H-indole-3-carbonitrile)
50 g of Compound 1 was dissolved in 1 l of acetonitrile and this solution was cooled to 5° C., and 36 ml (1.2 eq) of chlorosulfonylisocyanate was added dropwise. The temperature was raised to room temperature (25° C.) and the mixture was stirred for 2 hours. An excess of DMF was added, and the resulting mixture was stirred for 1 hour, after which the reaction was terminated with an excess of water, followed by performing layer separation with ethylacetate (EA), dehydrating with MgSO4, filtering and concentrating under reduced pressure, yielding 53.5 g (91.3%) of the title compound (Compound 2). 1H NMR (300 MHz, DMSO-d6, ppm, δ) 12.58 (bs, 1H), 10.20 (s, 1H), 8.31 (d, J=3.1 Hz, 1H), 8.03 (d, J=7.94 Hz, 1H), 7.97 (dd, J=7.72, J=1.13 Hz, 1H), 7.49 (t, 7.94 Hz, 1H)
References:
US2013/196972,2013,A1 Location in patent:Paragraph 0084-0085