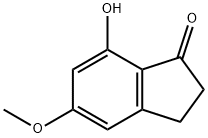
7-Hydroxy-5-Methoxy-2,3-dihydro-1H-inden-1-one synthesis
- Product Name:7-Hydroxy-5-Methoxy-2,3-dihydro-1H-inden-1-one
- CAS Number:76842-70-1
- Molecular formula:C10H10O3
- Molecular Weight:178.18
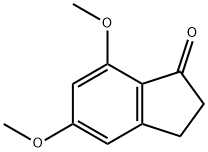
880-87-5

76842-70-1
General procedure for the synthesis of 7-hydroxy-5-methoxy-2,3-dihydro-1H-inden-1-one from 5,7-dimethoxyindanone: To a solution of 5,7-dimethoxyindanone (10.3 g, 54 mmol) in dichloromethane (100 mL) was slowly added a 1 M solution of boron trichloride (BCl3) (102 mL, 102 mmol) at 0 °C. The reaction system was kept at 0 °C and the resulting solution was stirred for 2.5 hours. Subsequently, the reaction mixture was slowly warmed to room temperature and stirring was continued for 2.5 hours. After completion of the reaction, the mixture was carefully poured into ice (100 g) to quench the reaction. The aqueous layer was extracted with dichloromethane and after combining the organic phases, the solvent was removed by distillation under reduced pressure to afford 7-hydroxy-5-methoxy-2,3-dihydro-1H-inden-1-one (9.3 g, 52 mmol) in 96% yield. The product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, CDCl3): δ 2.7 (t, 2H), 3.1 (t, 2H), 3.9 (s, 3H), 6.3 (m, 1H), 6.5 (m, 1H), 9.2 (bs, 1H); mass spectrometry (MS, ES) showed the molecular ion peak m/z 179 (M + 1)+.

880-87-5
62 suppliers
$91.00/250mg

76842-70-1
24 suppliers
$175.00/50mg
Yield:76842-70-1 96%
Reaction Conditions:
with boron trichloride in dichloromethane at 0 - 20; for 5 h;
Steps:
45.3
To a solution of 5, [7-DIMETHOXY-1-INDANONE] (10.3 g, 54 [MMOL)] in dichloromethane (100 mL) at [0C] was added 1 M solution of BCI3 (102 mL, 102 [MMOL)] in dichloromethane, and the resulting solution was stirred for 2.5 h at [0C.] The mixture was warmed to rt and stirred for 2.5 h. The reaction mixture was poured into 100 g ice, and the aqueous layer was extracted with dichloromethane. Solvents were evaporated under vacuum to obtain [7-HYDROXY-5-METHOXY-1-INDANONE] (9.3 g, 52 [MMOL)] in 96% [YIELD.'H] NMR [(CDCI3)] [B] 2.7 (t, 2H), 3.1 (t, 2H), 3.9 (s, 3H), 6.3 (m, [1H),] 6.5 (m, [1H),] 9.2 (bs, [1H)] ; MS (ES) 179 (M+1) [+.]
References:
WO2004/11446,2004,A1 Location in patent:Page 73
717-94-2
150 suppliers
$6.00/250mg

76842-70-1
24 suppliers
$175.00/50mg
16909-11-8
169 suppliers
$45.00/1g

76842-70-1
24 suppliers
$175.00/50mg
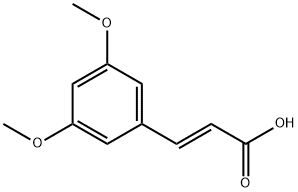
20767-04-8
16 suppliers
inquiry

76842-70-1
24 suppliers
$175.00/50mg
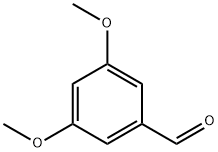
7311-34-4
329 suppliers
$10.00/1g

76842-70-1
24 suppliers
$175.00/50mg