
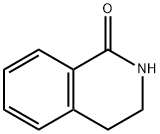
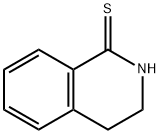
7-Iodo-3,4-dihydroisoquinolin-1(2H)-one synthesis
- Product Name:7-Iodo-3,4-dihydroisoquinolin-1(2H)-one
- CAS Number:66491-04-1
- Molecular formula:C9H8INO
- Molecular Weight:273.07
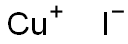
7681-65-4
577 suppliers
$6.00/5g
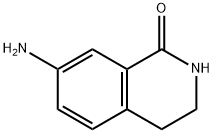
66491-03-0
84 suppliers
$74.00/100mg

66491-04-1
18 suppliers
inquiry
Yield:66491-04-1 640 mg
Reaction Conditions:
Stage #1: copper(l) iodide;7-amino-3,4-dihydroisoquinoline-1(2H)-onewith hydrogenchloride;sodium nitrite in water at 0; for 0.25 h;
Stage #2: with copper(l) iodide;potassium iodide in dichloromethane;water at 20;
Steps:
32 Preparation of Intermediate 7-Iodo-3,4-dihydro-2H-isoquinolin-1-one (I-32b)
Preparation of Intermediate 7-Iodo-3,4-dihydro-2H-isoquinolin-1-one (I-32b)
NaNO2 (340 mg, 4.9382 mmol) was added to a solution of 7-amino-3,4-dihydro-2H-isoquinolin-1-one (I-32a: 800 mg, 4.9382 mmol) in concentrated HCl (2 mL) and water (2 mL) at 0° C.
The reaction mixture was stirred at 0° C. for 15 minutes.
The resulting diazonium salt solution was added portion wise to a vigorously stirred biphasic mixture of DCM (25 mL), potassium iodide (4.9 g, 29.6242 mmol), copper iodide (47 mg, 0.25 mmol) and water (8 mL).
The resulting mixture was stirred at room temperature overnight.
The reaction was monitored by TLC (80% ethylacetate in hexane).
The reaction mixture was diluted with DCM.
The organic layer was washed with 10% Na2S2SO3 solution, dried over Na2SO4 and concentrated.
Purification by column chromatography on silica gel (50% ethylacetate in hexane) afforded 640 mg of the product (44.50% yield).
1H NMR (300 MHz, CDCl3): δ 8.53-8.31 (m, 1H), 7.80-7.70 (m, 1H), 7.0 (d, 1H), 6.40-6.25 (bs, 1H), 3.70-3.51 (m, 2H), 3.0 (t, 2H)
LCMS: 100%, m/z=274.0 (M+1)
References:
US2014/45872,2014,A1 Location in patent:Paragraph 0579

66491-03-0
84 suppliers
$74.00/100mg

66491-04-1
18 suppliers
inquiry
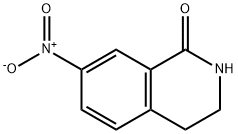
1196-38-9
167 suppliers
$8.00/100mg

66491-04-1
18 suppliers
inquiry
22245-96-1
63 suppliers
inquiry

66491-04-1
18 suppliers
inquiry
6552-60-9
18 suppliers
$137.75/1g

66491-04-1
18 suppliers
inquiry