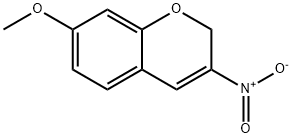
7-METHOXY-3-NITRO-2H-CHROMENE) synthesis
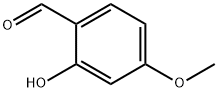
- Product Name:7-METHOXY-3-NITRO-2H-CHROMENE)
- CAS Number:92210-60-1
- Molecular formula:C10H9NO4
- Molecular Weight:207.18
Yield:92210-60-1 37%
Reaction Conditions:
with piperidine in toluene;Dean-Stark;Reflux;
Steps:
General procedure for the synthesis of 3-nitrosubstitutedchromenes
General procedure: To a stirred mixture of corresponding salicylilaldehyde(9 mmol) and 90 cm3 toluene in a 250-cm3 round bottomflask, equipped with a magnetic stir bar, 1.2 cm3 ethyl2-nitroacetate (10.8 mmol, 1.2 equiv) and 180 mm3piperidine (1.8 mmol, 0.2 equiv, 20 mol %) were added.The reaction mixture was refluxed for 24-48 h with stirring (reaction progress followed by TLC/HPLC). The generatedreaction water was removed by the means of a Dean-Starkapparatus to drive the reaction to completion. After thecorresponding reaction time finished, the resulting reactionmixture was cooled down at 0 C for 12 h, the precipitateseparated by filtration and washed with cold toluenefollowed by excess of cold n-heptane. The resulted productwas dried overnight and used without further purification inthe next synthetic step. Compounds 7 and 9 were obtainedvia automated flash chromatography with petroleum ether/ethyl acetate as eluent (0-10% EtOAc).
References:
Schwendt, Georg;Glasnov, Toma [Monatshefte fur Chemie,2017,vol. 148,# 1,p. 69 - 75]