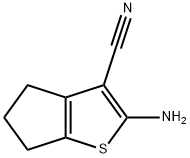
2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBONITRILE synthesis
- Product Name:2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBONITRILE
- CAS Number:70291-62-2
- Molecular formula:C8H8N2S
- Molecular Weight:164.23

120-92-3
431 suppliers
$11.00/25g

109-77-3
453 suppliers
$10.00/5g
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBONITRILE](/CAS/GIF/70291-62-2.gif)
70291-62-2
106 suppliers
$35.00/500mg
Yield:70291-62-2 90%
Reaction Conditions:
with morpholine;sulfur in ethanol at 30; for 8 h;Gewald Aminoheterocycles Synthesis;
Steps:
General procedure for the preparation of the substituted 2-aminothiophene-3-carbonitriles (1a-d)
General procedure: These compounds were prepared according to literature procedures with slight modification [19, 20]. Briefly, morpholine (5 mL, 60 mmol) was slowly added to a mixture of ketone (50 mmol), malonodinitrile (3.3 g, 50 mmol), and sulphur (1.6 g, 50 mmol) in ethanol (75 mL) at 30 °C. Then, the mixture was stirred for 8 h at this temperature. After completion, the reaction mixture was filtered, and the resulting filtrate was poured into cold water, and allowed to stand for 1 h. Finally, the precipitate was filtered, washed with cold aqueous ethanol (50%), and recrystallized from ethanol to give the desired Gewald product.
References:
Falanji, Farahnaz;Hosseyni-Tabar, Seyed Mahmood;Mahdavi, Behnam;Rezaei-Seresht, Esmail;Rezaei-Seresht, Hasan [Journal of the Iranian Chemical Society,2020,vol. 17,# 4,p. 809 - 815]

5660-83-3
10 suppliers
inquiry
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBONITRILE](/CAS/GIF/70291-62-2.gif)
70291-62-2
106 suppliers
$35.00/500mg

2235-08-7
1 suppliers
inquiry

120-92-3
431 suppliers
$11.00/25g
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBONITRILE](/CAS/GIF/70291-62-2.gif)
70291-62-2
106 suppliers
$35.00/500mg

120-92-3
431 suppliers
$11.00/25g
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBONITRILE](/CAS/GIF/70291-62-2.gif)
70291-62-2
106 suppliers
$35.00/500mg
![Spiro[cyclopentane-1,4'-[4H]indene]-5',5',7'(2'H)-tricarbonitrile,6'-amino-3',3'a-dihydro-](/CAS/20180808/GIF/21369-33-5.gif)