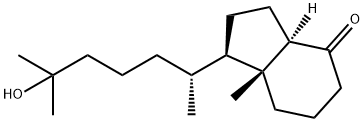
(1R,3aR,7aR)-1-((R)-6-hydroxy-6-Methylheptan-2-yl)-7a-Methylhexahydro-1H-inden-4(2H)-one synthesis
- Product Name:(1R,3aR,7aR)-1-((R)-6-hydroxy-6-Methylheptan-2-yl)-7a-Methylhexahydro-1H-inden-4(2H)-one
- CAS Number:70550-73-1
- Molecular formula:C18H32O2
- Molecular Weight:280.45
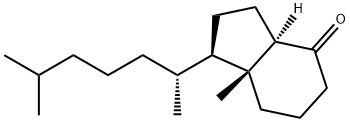
66251-18-1
19 suppliers
inquiry

70550-73-1
27 suppliers
inquiry
Yield:70550-73-1 82%
Reaction Conditions:
with potassium peroxymonosulfate sulfate;1,1,1-trifluoro-2-propanone;edetate disodium;sodium hydrogencarbonate in water;acetonitrile at 5; for 38 h;Darkness;
Steps:
8. (1R,3aR,7aR)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methy-loctahydro-4H-inden-4-one (28)4
A mixture of compound 27 (100 mg, 0.38 mmol), Na2EDTA (25 mg, 0.07 mmol) and H2O (250 uL) in MeCN (2.5 mL), in dark, 1,1,1-trifluoroacetone(0.5 mL, 5.6 mmol) was added followed by mixture of Oxone (424 mg, 0.69 mmol) and NaHCO3 (116 mg, 1.38 mmol) in small portions at 5 over a period of 6 h. Then the mixture of Oxone (600 mg, 0.97 mmol) and NaHCO3 (180 mg, 2.14 mmol) was added in three portions over a period of 32 h. The mixture was diluted with H2O and extracted with DCM. The organic layer was washed with brine, dried over anhydrous Na2SO4, ltered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc, 4:1) to give compound 28 (87 mg, 82%)
References:
Deng, Wutong;Gong, Yimou;Pu, Qingyin;Sun, Jian;Wang, Chao;Zhou, Li [Tetrahedron,2020] Location in patent:supporting information
66774-84-3
30 suppliers
inquiry

70550-73-1
27 suppliers
inquiry
![1H-Indene-1-pentanol, octahydro-4-hydroxy-α,α,ε,7a-tetramethyl-, [1R-[1α(R*),3aβ,4β,7aα]]- (9CI)](/CAS/20210305/GIF/132713-39-4.gif)
132713-39-4
0 suppliers
inquiry

70550-73-1
27 suppliers
inquiry
31944-51-1
0 suppliers
inquiry

70550-73-1
27 suppliers
inquiry