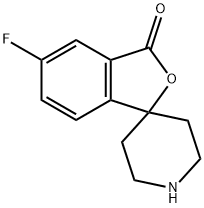
5-Fluoro-3H-spiro[isobenzofuran-1,4'-piperidin]-3-one synthesis
- Product Name:5-Fluoro-3H-spiro[isobenzofuran-1,4'-piperidin]-3-one
- CAS Number:707541-47-7
- Molecular formula:C12H12FNO2
- Molecular Weight:221.23
![Spiro[isobenzofuran-1(3H),4'-piperidin]-3-one, 5-fluoro-1'-(phenylmethyl)-](/CAS/20211123/GIF/644968-61-6.gif)
644968-61-6
0 suppliers
inquiry
![5-Fluoro-3H-spiro[isobenzofuran-1,4'-piperidin]-3-one](/CAS2/GIF/707541-47-7.gif)
707541-47-7
19 suppliers
$198.00/250mg
Yield:707541-47-7 96%
Reaction Conditions:
Stage #1: 1'-benzyl-5-fluoro-3H-spiro[2-benzofuran-1,4'-piperidine]-3-onewith hydrogenchloride;hydrogen;palladium 10% on activated carbon in ethanol;water; for 1 h;Reflux;
Stage #2: with sodium carbonate in water;
Steps:
1.b
1'-Benzyl-5-fluoro-3H-spiro[2-benzofuran-1,4'-piperidin]-3-one (0.622 g, 2 mmol) was suspended in ethanol (6 ml). The mixture was acidified with a few drops of HCl 37%. Pd/C 10% (65 mg, 0.06 mmol) was added. The mixture was refluxed under an hydrogen atmosphere for 1 hour then cooled to room temperature, purged with argon and diluted with dichloromethane. The catalyst was filtered and the filtrate was concentrated in vacuo. The white solid was dissolved in water (20 ml). The solution was basified with a 2M Na2CO3 solution and extracted 3 times with dichloromethane. The combined extracts were dried over Na2SO4, filtered and concentrated in vacuo to yield 0.42 g (96%) 5-fluoro-3H-spiro[2-benzofuran-1,4'-piperidin]-3-one as a white solid. MS m/e: 222 (M+H+).
References:
US2010/120751,2010,A1 Location in patent:Page/Page column 24