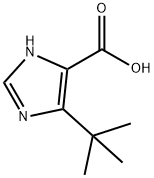
1H-Imidazole-4-carboxylicacid,5-(1,1-dimethylethyl)-(9CI) synthesis
- Product Name:1H-Imidazole-4-carboxylicacid,5-(1,1-dimethylethyl)-(9CI)
- CAS Number:714273-88-8
- Molecular formula:C8H12N2O2
- Molecular Weight:168.19

29509-07-7
1 suppliers
inquiry
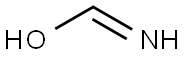
60100-09-6
0 suppliers
inquiry
51721-21-2
25 suppliers
$486.54/1g

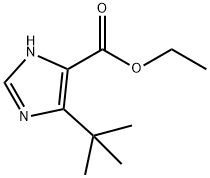
714273-89-9
35 suppliers
$65.00/100mg

714273-88-8
14 suppliers
$356.00/500mg
Yield:51721-21-2 26% ,714273-89-9 26% ,714273-88-8 2%
Reaction Conditions:
in water at 150; for 3.5 h;
Steps:
3.h 5-tert-Butyl-3H-imidazole-4-carboxylic acid ethyl ester
A solution of 2-chloro-4,4-dimethyl-3-oxo-pentanoic acid ethyl ester (25.0 g, 0.12 mol) in formamide (47.5 ml) and water (2.5 ml) was shaken, then dispensed into 15×8 ml vials. All vials were sealed and then heated at 150° for 3.5 h. The vials were allowed to cool to room temperature, then water (20 ml) was added and the mixture was exhaustively extracted with chloroform. The chloroform was removed to give a concentrated formamide solution (22.2 g) which was added to a flash silica column (6 cm diameter, 12 cm height) packed in 1% MeOH/1% Et3N in chloroform. Elution of the column with 2.5 L of this mixture followed by 1 L of 2% MeOH/1% Et3N in chloroform gave, in the early fractions, a product suspected of being 5-tert-butyl-oxazole-4-carboxylic acid ethyl ester (6.3 g, 26%).HPLC (214 nm) tR=8.77 min.1H NMR (400 MHz, CDCl3) δ 1.41 (t, J=7.2 Hz, 3H); 1.43 (s, 9H); 4.40 (q, J=7.2 Hz, 2H); 7.81 (s, 1H).13C NMR (100 MHz, CDCl3) δ 14.1, 28.8, 32.5, 61.3, 136.9, 149.9, 156.4, 158.3.ESMS m/z 198.3 [M+H]+, 239.3 [M+CH4CN].LC/MS tR=7.97 (198.1 [M+H]+) min.Recovered from later fractions was 5-tert-butyl-3H-imidazole-4-carboxylic acid ethyl ester (6.20 g, 26%). (Durant et al., “Aminoalkylimidazoles and Process for their Production.” Patent No. GB 1341375 (Great Britain, 1973)).HPLC (214 nm) tR=5.41 (93.7%) min.1H NMR (400 MHz, CDCl3) δ 1.38 (t, J=7.0 Hz, 3H); 1.47 (s, 9H); 4.36 (q, J=7.2 Hz, 2H); 7.54 (s, 1H).13C NMR (100 MHz, CDCl3) δ 13.7, 28.8, 32.0, 59.8, 124.2, 133.3, 149.2, 162.6.ESMS m/z 197.3 [M+H]+, 238.3 [M+CH4CN].Further elution of the column with 1 L of 5% MeOh/1% Et3N gave a compound suspected of being 5-tert-butyl-3H-imidazole-4-carboxylic acid (0.50 g, 2%).HPLC (245 nm) tR=4.68 (83.1%) min.1H NMR (400 MHz, CD3OD) δ 1.36 (s, 9H); 7.69 (s, 1H).1H NMR (400 MHz, CDCl3) δ 1.37 (s, 9H); 7.74 (s, 1H).1H NMR (400 MHz, CD3SO) δ 1.28 (s, 9H); 7.68 (s, 1H).ESMS m/z 169.2 [M+H]+, 210.4 [M+CH4CN].
References:
US2008/221122,2008,A1 Location in patent:Page/Page column 27
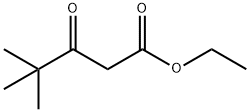
17094-34-7
114 suppliers
$21.00/5mL

714273-89-9
35 suppliers
$65.00/100mg

714273-88-8
14 suppliers
$356.00/500mg