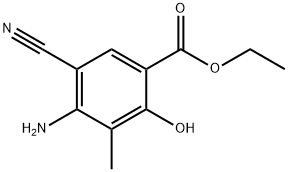
Benzoic acid, 4-aMino-5-cyano-2-hydroxy-3-Methyl-, ethyl ester synthesis
- Product Name:Benzoic acid, 4-aMino-5-cyano-2-hydroxy-3-Methyl-, ethyl ester
- CAS Number:72817-85-7
- Molecular formula:C11H12N2O3
- Molecular Weight:220.22
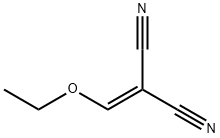
123-06-8
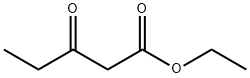
4949-44-4

72817-85-7
Example 74 Synthesis of ethyl 4-amino-5-cyano-2-hydroxy-3-methylbenzoate (74): ethyl propionylacetate (25 g, 0.17 mol) was slowly added to a freshly prepared sodium ethanol solution (prepared by reacting sodium metal (7.9 g, 0.35 mol) with ethanol (1.3 L)) at 0 °C, followed by stirring the reaction mixture for 1 hour at room temperature. Next, ethoxymethylene malononitrile (21 g, 0.17 mol) was added to the above reaction system at room temperature and the reaction mixture was warmed up to 80°C and refluxed for 2 hours. Upon completion of the reaction, the mixture was cooled to room temperature, neutralized to pH=7 with 1.5N HCl solution and subsequently concentrated under vacuum. The concentrated residue was diluted with 100 mL of water and the solid product was collected by filtration. The solid product was washed with water and dried under vacuum at 50 °C to give a crude product of 27 g. The crude product was further purified by washing with petroleum ether containing 5% ethyl acetate to give a final pure title compound of 22.5 g in 59% yield.
Yield:72817-85-7 59%
Reaction Conditions:
Stage #1: ethyl propanoylacetatewith sodium ethanolate in ethanol at 0 - 20;
Stage #2: Ethoxymethylenemalononitrile in ethanol at 20 - 80; for 2 h;
Stage #3: with hydrogenchloride;water in ethanol; pH=7;
Steps:
74
Example 74 4-Amino-5-cyano-2-hydroxy-3-methylbenzoic acid ethyl ester (74) To a solution of sodium ethoxide (1.3 L) (freshly prepared by addition of sodium metal (7.9 g, 0.35 mol) to ethanol (1.3 L)) at 0° C. was added ethylpropionyl acetate (25 g, 0.17 mol) and the solution was stirred at RT for 1 h. To the above solution was added ethoxymethylene malonormitrile (21 g, 0.17 mol) at RT and the reaction mixture was refluxed at 80° C. for 2 h. The reaction mixture was cooled, neutralized to pH=7 by addition of 1.5 N HCl and concentrated under vacuum. The obtained residue was diluted with water (100 mL) and filtered. The solid was washed with water and dried under vacuum at 50° C. to give the crude product (27 g). The crude solid was washed with 5% ethyl acetate in pet. ether which gave pure title compound (22.5 g, 59%).
References:
US2009/118312,2009,A1 Location in patent:Page/Page column 52

123-06-8
351 suppliers
$14.14/1gm:

72817-85-7
23 suppliers
inquiry