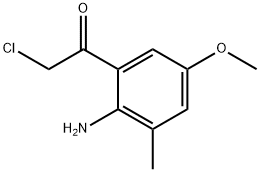
Ethanone, 1-(2-amino-5-methoxy-3-methylphenyl)-2-chloro- (9CI) synthesis
- Product Name:Ethanone, 1-(2-amino-5-methoxy-3-methylphenyl)-2-chloro- (9CI)
- CAS Number:74798-63-3
- Molecular formula:C10H12ClNO2
- Molecular Weight:213.66
Yield:74798-63-3 55.2%
Reaction Conditions:
Stage #1: chloroacetonitrile;2-methyl-4-methoxyanilinewith boron trichloride;titanium tetrachloride in dichloromethane;toluene at -78 - 90; for 3 h;
Stage #2: with hydrogenchloride in dichloromethane;water;toluene at 85; for 0.75 h;
Steps:
1.1 The first step: 1-(2-amino-5-methoxy-3-methylphenyl)-2-chloroethanone (1b)
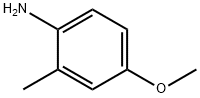
2-Methyl-4-methoxyaniline (1a) (5.0 g, 36.4 mmol) was dissolved in toluene (20 mL), and boron trichloride (37 mL, 1M dichloromethane solution) was added successively at -78°C, Titanium tetrachloride (37 mL, 1M in dichloromethane) and chloroacetonitrile (3.1 g, 41 mmol). The reaction solution was heated to 90°C and stirred for 3h. A 2M aqueous hydrochloric acid solution (30 mL) was added to the reaction system, and the mixture was stirred at 85° C. for 45 min. Saturated sodium bicarbonate solution (100 mL) was added to the reaction system, followed by extraction with dichloromethane (100 mL×3). The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate:petroleum ether (v/v)=1:5) to obtain a yellow solid 1-(2-amino- 5-Methoxy-3-methylphenyl)-2-chloroethanone (1b) (4.30 g, yield: 55.2%).
References:
CN113880747,2022,A Location in patent:Paragraph 0113-0119