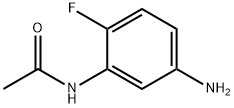
N-(5-amino-2-fluorophenyl)acetamide(SALTDATA: 0.95HCl 0.8H2O) synthesis
- Product Name:N-(5-amino-2-fluorophenyl)acetamide(SALTDATA: 0.95HCl 0.8H2O)
- CAS Number:75001-47-7
- Molecular formula:C8H9FN2O
- Molecular Weight:168.17
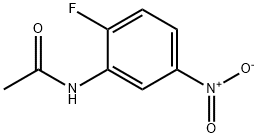
454-07-9
17 suppliers
inquiry

75001-47-7
24 suppliers
$98.00/1g
Yield:75001-47-7 99%
Reaction Conditions:
with palladium 10% on activated carbon;ammonium formate in methanol;water at 20;
Steps:
7.B Step B
The title compound from Step A above (4.45 g, 22.5 mmol) was dissolved in methanol (80 ml_) and ammonium formate was added (7.08 g, 112.5 mmol). After the addition of a suspension of 10 % palladium on charcoal (0.71 g) in water (7 ml_), the mixture was stirred at room temperature overnight. The reaction mixture was filtered and the catalyst was washed withmethanol (15 mL). The filtrate was evaporated under reduced pressure and the residue was re- dissolved in ethyl acetate (200 mL) and water (50 mL). The organic phase was separated, dried over Na2S04, filtered and the solvent was removed under reduced pressure to afford the title compound as an off-white solid (3.77 g, 99 %). 1H-NMR (400 MHz, CDCI3): δ = 2.23 (s, 3H), 3.63 (br-s, 2H), 6.33-6.37 (m, 1 H), 6.88 (t, 1 H), 7.34 (br-s, 1 H), 7.76-7.78 (m, 1 H)
References:
WO2015/110263,2015,A1 Location in patent:Page/Page column 142; 143
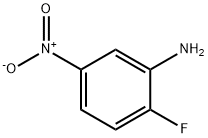
369-36-8
257 suppliers
$10.00/10g

75001-47-7
24 suppliers
$98.00/1g