
1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl- synthesis
- Product Name:1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl-
- CAS Number:75821-80-6
- Molecular formula:C22H30N4O
- Molecular Weight:366.5
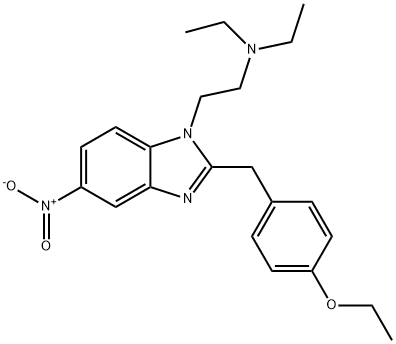
911-65-9
0 suppliers
$82.00/1mg
![1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl-](/CAS/20210305/GIF/75821-80-6.gif)
75821-80-6
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20; for 3 h;
Steps:
1
Preparation of 10: {2-[2-(4-Ethoxy-benzyl)-5-nitro-benzoimidazol-1-yl]-ethyl}-diethyl-amine 7a (150 mg, 0.378 mmol) was dissolved in anhydrous ethanol (10 mL) in a dry argon purged flask. Palladium, 10 wt % on activated carbon, (40.3 mg, 0.0378 mmol) is quickly added and the atmosphere from the flask evacuated by vacuum pump and replaced with hydrogen from a balloon. The atmosphere is evacuated from the flask and replaced with hydrogen twice more and the mixture stirred under a hydrogen atmosphere at room temperature. After 3 hours, thin layer chromatography in a solvent system of (5% 2M NH3 in methanol/95% dichloromethane) showed complete conversion to 8a 1-(2-Diethylamino-ethyl)-2-(4-ethoxy-benzyl)-1H-benzoimidazol-5-ylamine, which is utilized without isolation. The mixture is filtered through a pad of celite to remove insolubles, the pad washed with anhydrous ethanol (10 mL) and the ethanolic solution of the amine 8a is charged to a small, argon purged flask fitted with a magnetic stirbar. Thiophene-2-carboximidothioic acid methyl ester hydroiodide 9a (140 mg, 0.491 mmol) is added to the flask and the reaction was stirred under Ar at ambient temperature for 67 hours. The solution was diluted with diethyl ether (80 ml) and cooled in and ice bath resulting in the formation of an off-white precipitate that was collected on a sintered glass funnel and washed with ether. The hygroscopic solid was solubilized on the funnel in methanol and the solvent collected and evaporated to yield crude solid. The solid was partitioned between H2O and ethyl acetate and 1M sodium hydroxide solution added to adjust pH to 8. The mixture was transferred to a separatory funnel and the organic layer collected. The aqueous layer was further extracted with ethyl acetate and the combined organic layers were washed with brine (twice), dried over magnesium sulphate, filtered and concentrated to afford yellow residue 10 N-[1-(2-Diethylamino-ethyl)-2-(4-ethoxy-benzyl)-1H-benzo-imidazol-5-yl]-thiophene-2-carboxamidine. Yield: 94 mg (52.2%). 1H NMR (DMSO) d: 0.82 (t, 6H, J=7.1), 1.29 (t, 3H, J=7.0), 2.45 (q, 4H, J=7.0), 2.45-2.50 (m, 2H), 3.98 (q, 2H, J=7.0), 4.07-4.13 (m, 2H), 4.22 (s, 2H), 6.31 (br s, 2H), 6.72 (d, 1H, J=7.7), 6.88 (d, 2H, J=8.7), 6.99 (s, 1H), 7.08-7.11 (m, 1H), 7.17 (d, 2H, J=8.4), 7.35 (d, 1H, J=8.4), 7.59 (d, 1H, J=5.1), 7.72 (d, 1H, J=3.4); MS (ESI): 476 (MH+, 100%)
References:
US2008/214613,2008,A1 Location in patent:Page/Page column 40-41
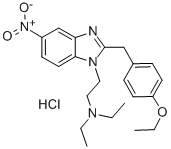
2053-25-0
0 suppliers
inquiry
![1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl-](/CAS/20210305/GIF/75821-80-6.gif)
75821-80-6
7 suppliers
inquiry
![1-N-[2-(DIETHYLAMINO)ETHYL]-4-NITROBENZENE-1,2-DIAMINE](/CAS/20180808/GIF/5099-39-8.gif)
5099-39-8
4 suppliers
inquiry
![1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl-](/CAS/20210305/GIF/75821-80-6.gif)
75821-80-6
7 suppliers
inquiry
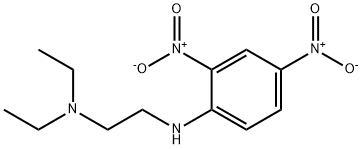
56223-91-7
9 suppliers
$50.00/50mg
![1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl-](/CAS/20210305/GIF/75821-80-6.gif)
75821-80-6
7 suppliers
inquiry
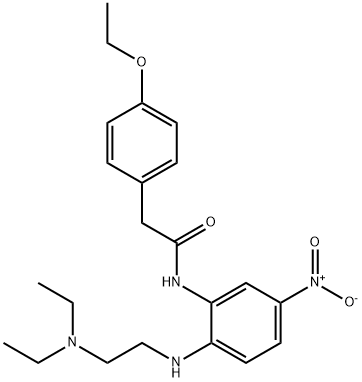
55154-71-7
1 suppliers
inquiry
![1H-Benzimidazole-1-ethanamine, 5-amino-2-[(4-ethoxyphenyl)methyl]-N,N-diethyl-](/CAS/20210305/GIF/75821-80-6.gif)
75821-80-6
7 suppliers
inquiry