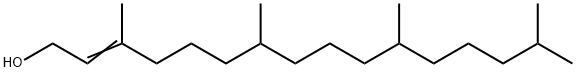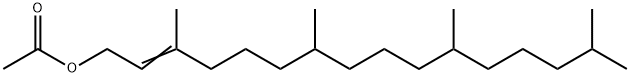
2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, 1-acetate synthesis
- Product Name:2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, 1-acetate
- CAS Number:76337-16-1
- Molecular formula:C22H42O2
- Molecular Weight:338.57
Yield:76337-16-1 134 g
Reaction Conditions:
with magnesium(II) perchlorate at 15; for 7 h;Reagent/catalyst;Temperature;
Steps:
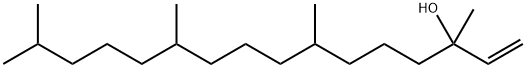
1-10 Example 5: Synthesis of 3,7,11,15-tetramethylhexadec-2-enyl acetate
In the reaction kettle, add acetic anhydride (122.4 g, 1.2 mol) and magnesium perchlorate (0.89 g, 4 mmol) in sequence, and stir and mix well. The temperature was lowered to 15 °C, and isophytol (118.4g, 0.4mol) was started to be added dropwise for 2 h and then the reaction was kept at 15 °C for 5 h. After the reaction is(the area normalized content of the raw material isophytoalcohol in GC analysis is less than or equal to 0.5%), add 200 mL of water and 500 mL of n-hexane and stir for 0.5 h. Extract and wash, stand still for stratification, and then wash twice with 400 mL of water. After the organic layer was decompressed to recover the n-hexane, 134.0 g of the intermediate phytoacetate was obtained, with a molar yield of 94.9%, and GC analysis showed a purity of 95.8%.
References:
CN112500287,2021,A Location in patent:Paragraph 0042-0061
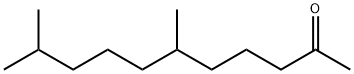
1604-34-8
24 suppliers
inquiry

76337-16-1
1 suppliers
inquiry
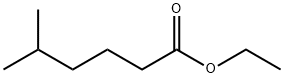
10236-10-9
12 suppliers
inquiry

76337-16-1
1 suppliers
inquiry