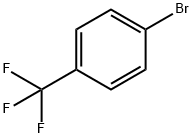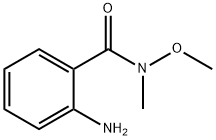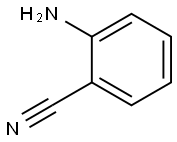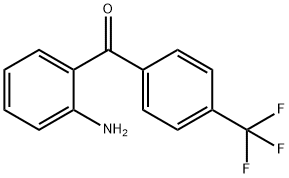
(2-aminophenyl)(4-(trifluoromethyl)phenyl)methanone synthesis
- Product Name:(2-aminophenyl)(4-(trifluoromethyl)phenyl)methanone
- CAS Number:790-10-3
- Molecular formula:C14H10F3NO
- Molecular Weight:265.23
![Acetamide, N-[2-[4-(trifluoromethyl)benzoyl]phenyl]-](/CAS/20210305/GIF/120301-10-2.gif)
120301-10-2
0 suppliers
inquiry

790-10-3
8 suppliers
inquiry
Yield:790-10-3 90%
Reaction Conditions:
Stage #1: N-(2-(4-(trifluoromethyl)benzoyl)phenyl)acetamidewith hydrogenchloride in water;acetone at 70; for 2 h;
Stage #2: with potassium carbonate in water;acetone;
Steps:
2 4.4.1. General procedure for the syntheses of 2-aminobenzophenones 2
General procedure: To a solution of the corresponding 39 acetamide 1a-c in 40 acetone (0.15m), aqueous 6m 41 HCl (40eq.) was added. The solution was stirred at 70°C for 2h followed by the addition of 42 K2CO3 until basic. The crude amine was extracted with CH2Cl2 and the organic layer dried over Na2SO4 and then concentrated in vacuo after filtration. The resulting 2-aminobenzophenones 43 2a-c were used without further purification. 4.4.1.2
2-Amino-4'-(trifluoromethyl)benzophenone (2b)
90% yield. 1H NMR δ 6.25 (2H, brs), 6.64 (ddd, J = 8.1, 7.6, 1.1 Hz), 6.79 (dd, J = 8.3, 0.6 Hz), 7.33-7.41 (2H, m), 7.76 (4H, s).
13C NMR δ 115.6, 117.2, 123.9 (qt, 1JF,C = 272.6 Hz), 125.2 (qt, 3JF,C = 3.6 Hz), 127.4, 129.1, 132.4 (qt, 2JF,C = 32.5 Hz), 134.4, 134.9, 143.5, 151.4, 197.7. 19F NMR δ -62.8. HRMS: calcd for C14H11F3NO [M+H]+ 266.0793, found 266.0799.
References:
Han, Jianlin;Ace?a, José Luis;Yasuda, Nobuhiro;Uekusa, Hidehiro;Ono, Taizo;Soloshonok, Vadim A.;Klika, Karel D. [Inorganica Chimica Acta,2015,vol. 433,p. 3 - 12]
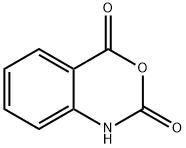
118-48-9
430 suppliers
$5.00/5G

790-10-3
8 suppliers
inquiry
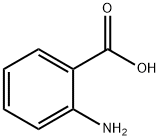
118-92-3
0 suppliers
$23.00/250g

790-10-3
8 suppliers
inquiry