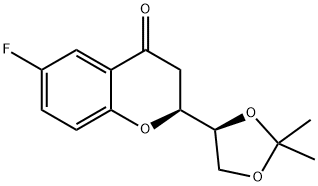
(1’S,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one synthesis
- Product Name:(1’S,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one
- CAS Number:797054-20-7
- Molecular formula:C14H15FO4
- Molecular Weight:266.26
797054-17-2
8 suppliers
$155.00/500mg
![(1’S,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-20-7.gif)
797054-20-7
7 suppliers
$555.00/1g
![(1’S,2R)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-21-8.gif)
797054-21-8
5 suppliers
$120.00/100mg
Yield:797054-20-7 28.2% ,797054-21-8 21%
Reaction Conditions:
with lithium hydroxide in water at 60; for 1 h;
Steps:
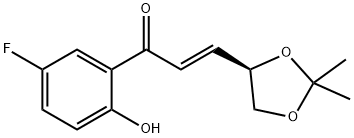
11 Example 11; (S,S)-2-(2',2'-dimethyl-[1,3]-dioxolane-4-yl)-6-fluoro-chroman-4-one and (R,S)-2-(2',2'-dimethyl-[1,3]-dioxolane-4-yl)-6-fluoro-chroman-4-one [S,S) and X (R,S)]
51.6 g (0,19 moles) of (E)- (SR)-4, 5-ISOPROPYLYDENE-DIOXY-1- (2-HYDROXY-5-FLUORO-PHENYL)-PROP-2E-ENE-L-ONE is suspended in 65 ml of water at 60°C, then 48 mg (0,0052 moles) of lithium hydroxide is added to the reaction mixture and stirred at 60°C for an hour. The reaction mixture is allowed to cool to 35°, 100 ml of diethyl ether and 2,5 ml of ethanol is added to the reaction mixture. The layers are separated, the organic layer is dried, and the solvent is evaporated in vacuum. The obtained oily 26 g oily product is separated by vacuum chromatography. Eluents are increasing polarity mixtures of diethyl ether and petrolether (40-70°C). Acceptable fractions are collected, evaporated. The obtained 10,2 g (28,2%) of (S, S)-2- (2', 2'-dimethyl- [1, 3] -dioxolane-4-yl) -6-fluoro-chroman-4-one is recrystallised from n- hexane. Mp.: 62-63°C. Optical rotation is [A] D20 (C=1, CHC13) = +58, 00. Continued the chromatographic process, the acceptable fractions are collected, evaporated. The obtained 5,6 g (21%) of (R, S)- 2- (2', 2'-DIMETHYL- [1, 3]-DIOXOLANE-4-YL)-6-FLUORO-CHROMAN-4-ONE is recrystallised from n-hexane. Mp.: 42, 5-45°C. Optical rotation is [AD20 (C=1, CHC13) =-57, 20.
References:
WO2004/41805,2004,A1 Location in patent:Page 49