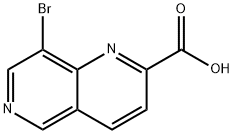
8-BROMO-1,6-NAPHTHYRIDINE-2-CARBOXYLIC ACID synthesis
- Product Name:8-BROMO-1,6-NAPHTHYRIDINE-2-CARBOXYLIC ACID
- CAS Number:197507-55-4
- Molecular formula:C9H5BrN2O2
- Molecular Weight:253.05
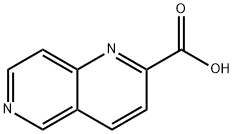
197507-59-8
110 suppliers
$13.00/100mg

197507-55-4
64 suppliers
$99.00/500mg
Yield:197507-55-4 2.9 g
Reaction Conditions:
with bromine;acetic acid at 80; for 4 h;
Steps:
To a stirred solution of 2g (11.48 mmol) of 1, 6-naphthyridine-2-carboxylic acid (CAS 07-59-8) in 50 mL of AcOH was added 1.18 mL (22.97 mmol) of Br2 dropwise. The resulting mixture was stirred at 80°C for 4 h. The mixture was concentrated under vacuum. The residue was washed with Et20 to afford 2.9 g of 8- bromo-1, 6-naphthyridine-2-carboxylic acid as a brown solid.To a solution of 2.4 g (9.48 mmol) of 8-bromo-1, 6-naphthyridine-2-carboxylic acid in 50 mL of DMF were added 4.64 g (14.2 mmol) of Cs2CO3 and 0.89 mL (14.2 mmol) of Mel. The resulting mixture was stirred at 30°C for 5 h. The mixture was diluted with 50 mL of water and extracted with 150 mL (50 mL*3) of EA. The combined organic layers were dried over Na2SO4, concentrated under vacuum. Theresidue was purified by silica gel column chromatography (DCMIMeOH = 100/2) to afford 2.3 g of methyl 8-bromo-1, 6-naphthyridine-2-carboxylate as a yellow solid.Under argon atmosphere, to a stirred solution of 3.5 g (13.1 mmol) of methyl 8-bromo-1, 6- naphthyridine-2-carboxylate in 110 mL of DMF/H20 (v/v = 10/1) were added 2.55g (15.7 mmol) of benzofuran-2-yl boronic acid (CAS 98437-24-2), 5.56 g (26.21 mmol) of K3P04.3H20 and 2.27 g (1.97mmol) of Pd(PPh3)4. After stirring at 80 °C for 3 hours, the mixture was diluted with water and extracted with EA. The separtaed organic layer was dried over Na2SO4, concentrated under vacuum. The residue was purified by silica gel column chromatography (PE/EA = 2/1) to afford 2.11 g of methyl 8- (benzofuran-2-yl)-1, 6-naphthyridine-2-carboxylate as a yellow solid.To a stirred solution of 1.8 g (5.92 mmol) of methyl 8-(benzofuran-2-yl)-1, 6-naphthyridine-2-carboxylatein 40 ml. of THF/H20 (v/v = 1/1) was added 0.99 g (23.66 mmol) of LiOH.H20. The resulting mixturewas stirred at 20°C for 1 h. The mixture was extracted with 120 mL (40 mLx3) of DCM/i-PrOH (v/v = 3/1). The combined organic layers were concentrated under vacuum. The residue was washed with Et20 to give 1.21 g of 8-(benzofuran-2-yl)- 1, 6-naphthyridine-2-carboxylic acid as a yellow solid. MS (ESI+): 291.0 [M+H].‘H NMR (400 MHz, DMSO-d6) ppm: 13.98 (br, 1H), 9.53 (s, 1H), 9.44 (s, 1H), 8.89 (d, J 8.4 Hz,1H), 8.56 (s, 1H), 8.38 (d, J= 8.4 Hz, 1H), 7.82 (d, J= 7.6 Hz, 1H), 7.73 (d, J= 8.4 Hz, 1H), 7.45-7.41 (m, 1H), 7.35-7.3 1 (m, 1H).
References:
BASILEA PHARMACEUTICA AG;POHLMANN, Jens;STIEGER, Martin;REINELT, Stefan;LANE, Heidi WO2016/128465, 2016, A1 Location in patent:Page/Page column 91; 92
98400-69-2
171 suppliers
$6.00/1g

197507-55-4
64 suppliers
$99.00/500mg
42373-30-8
172 suppliers
$23.00/500mg

197507-55-4
64 suppliers
$99.00/500mg
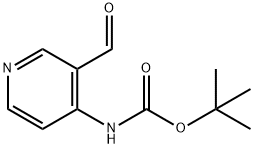
116026-93-8
87 suppliers
$70.00/250 mg

197507-55-4
64 suppliers
$99.00/500mg