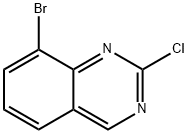
8-BROMO-2-CHLOROQUINAZOLINE synthesis
- Product Name:8-BROMO-2-CHLOROQUINAZOLINE
- CAS Number:956100-63-3
- Molecular formula:C8H4BrClN2
- Molecular Weight:243.49
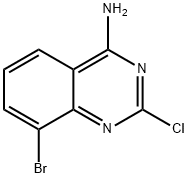
956100-62-2

956100-63-3
General procedure for the synthesis of 8-bromo-2-chloroquinazoline from 4-amino-8-bromo-2-chloroquinazoline: 8-bromo-2-chloroquinazolin-4-amine (12.93 g, 50 mmol) was dissolved in THF (500 mL) under stirring conditions, and isoamyl nitrite (23.43 g, 200 mmol) was added slowly dropwise over a dropwise period of 3 hours. Subsequently, the reaction mixture was continued to be stirred at 60 °C for 40 min. Upon completion of the reaction, the progress of the reaction was monitored by TLC (unfolding agent: PE:EA = 1:1). When the reaction was complete, the mixture was cooled to room temperature and the solvent was removed by distillation under reduced pressure. The residue was dissolved in DCM (300 mL) and the organic layer was washed sequentially with brine (100 mL) and water (100 mL). The organic layer was dried over anhydrous Na2SO4 and purified by column chromatography (eluent: DCM:PE = 1:1) to afford the target compound 8-bromo-2-chloroquinazoline as a yellow solid (8.21 g, 67% yield).1H NMR (DMSO-d6, 400 MHz): δ 9.66 (s, 1H), 8.45 (dd, J = 0.8, 7.6 Hz, 1H), 8.26 (dd, J = 0.8, 8.0 Hz, 1H), 7.73 (dd, J = 7.6, 8.0 Hz, 1H).

956100-62-2
79 suppliers
$27.00/1g

956100-63-3
122 suppliers
$55.00/250mg
Yield: 67%
Reaction Conditions:
with isopentyl nitrite in tetrahydrofuran at 60; for 8.67 h;
Steps:
L; 195d
To a stirred mixture of 8-bromo-2-chloroquinazolin-4-amine (12.93 g, 50 mmol) in THF (500 mL) was added isoamyl nitrite (23.43 g, 200 mmol) over 3 hours and 40 mins at 6O0C. The mixture was stirred for another 5 hours. TLC (PE:EA,1 :1) showed reaction complete. After cooling to room temperature, the
References:
PFIZER PRODUCTS INC. WO2007/125405, 2007, A2 Location in patent:Page/Page column 25; 81-82
331647-05-3
122 suppliers
$18.00/100mg

956100-63-3
122 suppliers
$55.00/250mg
20776-51-6
263 suppliers
$6.00/1g

956100-63-3
122 suppliers
$55.00/250mg
331646-99-2
82 suppliers
$11.00/250mg

956100-63-3
122 suppliers
$55.00/250mg

331646-99-2
82 suppliers
$11.00/250mg

956100-63-3
122 suppliers
$55.00/250mg