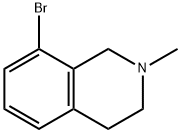
8-bromo-2-methyl-1,2,3,4-tetrahydroisoquinoline synthesis
- Product Name:8-bromo-2-methyl-1,2,3,4-tetrahydroisoquinoline
- CAS Number:947499-03-8
- Molecular formula:C10H12BrN
- Molecular Weight:226.11
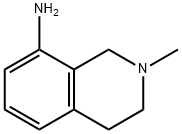
14788-34-2
6 suppliers
inquiry

947499-03-8
9 suppliers
inquiry
Yield:947499-03-8 65%
Reaction Conditions:
Stage #1: 2-methyl-1,2,3,4-tetrahydro-isoquinolin-8-ylaminewith hydrogen bromide;copper(I) bromide;sodium nitrite in water at 0; for 3.16667 h;
Stage #2: with sodium hydroxide in water; pH=9;
Steps:
4 Synthesis of 8-bromo-2-methyl-1,2,3,4-tetrahydroisoquinoline
Synthesis of 8-bromo-2-methyl-1,2,3,4-tetrahydroisoquinoline Into a 50 mL 3-necked round-bottom flask (named A), was placed 2-methyl-1,2,3, tetrahydroisoquinolin-8-amine (500 mg, 3.08 mmol). This was followed by the addition of a solution of HBr (5 mL) in H2O (5 mL), which was added dropwise with stirring, while cooling to a temperature of 0° C. To the above was added NaNO2 (230 mg, 3.33 mmol) in several batches, while cooling to a temperature of 0° C. and the mixture was stirred for 30 mins at that temperature. Then into another 50 mL 3-necked round-bottom flask (named B), was purged and maintained with an inert atmosphere of nitrogen, was placed a solution of CuBr (550 mg, 3.83 mmol) in HBr/H2O (3 mol/L) (10 mL), while cooling to a temperature of 0° C. The mixture was stirred for 10 min. Then was followed by the addition of the reaction solution of flask A with dropwise while the temperature was maintained at 0° C. The resulting solution was allowed to react, with stirring, for 30 mins while the temperature was maintained at 0° C. The resulting solution was allowed to react, with stirring, for an additional 2 h while the temperature was maintained at room temperature. The reaction progress was monitored by TLC(EtOAc:PE=1:1). Adjustment of the pH to 9 was accomplished by the addition of NaOH (10%). The resulting solution was extracted three times with 50 mL of CH2Cl2 and the organic layers combined and dried over K2CO3. A filtration was performed. The filtrate was concentrated by evaporation under vacuum using a rotary evaporator. The residue was purified by eluding through a column with a 1:1 PE:AE solvent system. This resulted in 0.45 g (65%) of 8-bromo-2-methyl-1,2,3,4-tetrahydroisoquinoline as a light yellow oil.
References:
US2008/318941,2008,A1 Location in patent:Page/Page column 23