
8-BroMo-3,7-difluoro-2-Methoxy-1,5-naphthyridine synthesis
- Product Name:8-BroMo-3,7-difluoro-2-Methoxy-1,5-naphthyridine
- CAS Number:943025-91-0
- Molecular formula:C9H5BrF2N2O
- Molecular Weight:275.05
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

943025-91-0
13 suppliers
$503.94/5MG
Yield: 57%
Reaction Conditions:
Stage #1:8-hydroxy-3,7-difluoro-2-(methyloxy)-1,5-naphthyridine with phosphorus tribromide in N,N-dimethyl-formamide for 1.5 h;
Stage #2: with potassium carbonate in water;N,N-dimethyl-formamideAlkaline conditions;
Steps:
9.f
A solution of 1,1 -dimethylethyl [2-[3-(dimethylamino)-2-fluoroacryloyl]-5- fluoro-6-(methyloxy)-3-pyridinyl]carbamate (2.15g, 6.02 mmol) in trifluoroacetic acid/dichloromethane (20 ml/20 ml) was stirred at room temperature for 0.5 hour under argon and evaporated. The residue was treated with ~50ml 2M ammonia in methanol until basic and evaporated. This residue was dried in vacuo then chromatographed eluting with 0-10% methanol in ethyl acetate affording a solid (3.64g), 8-hydroxy-3,7-difluoro-2- (methyloxy)-l,5-naphthyridine. 8-Hydroxy-3,7-difluoro-2-(methyloxy)-l,5-naphthyridine (3.64g) was dissolved in N,N-dimethylformamide (20 ml) and treated with phosphorus tribromide (0.68 ml, 7.22 mmol). After 1 hour, more phosphorus tribromide (0.68 ml, 7.22 mmol) was added. After 0.5 hour the mixture was diluted with water and basified with solid potassium carbonate. Filtration and drying in vacuo afforded the product (0.95g, 57%).MS (+ve ion electrospray) m/z 275/277 (MH+).
References:
GLAXO GROUP LIMITED WO2008/128962, 2008, A1 Location in patent:Page/Page column 57
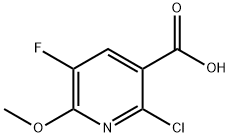
943025-86-3
41 suppliers
$30.00/100mg

943025-91-0
13 suppliers
$503.94/5MG

943025-87-4
5 suppliers
inquiry

943025-91-0
13 suppliers
$503.94/5MG
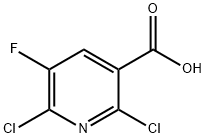
82671-06-5
313 suppliers
$5.00/1g

943025-91-0
13 suppliers
$503.94/5MG
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

943025-91-0
13 suppliers
$503.94/5MG