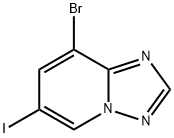
8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine synthesis
- Product Name:8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine
- CAS Number:1415314-10-1
- Molecular formula:C6H3BrIN3
- Molecular Weight:323.92
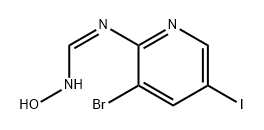
1415728-06-1
0 suppliers
inquiry
![8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine](/CAS/20180703/GIF/1415314-10-1.gif)
1415314-10-1
8 suppliers
inquiry
Yield:1415314-10-1 62%
Reaction Conditions:
Stage #1: (E)-N'-(3-bromo-5-iodopyridin-2-yl)-N-hydroxy-formimidamidewith trifluoroacetic anhydride in tetrahydrofuran at 0 - 20;
Stage #2: with sodium hydrogencarbonate in tetrahydrofuran;water; pH=8;
Steps:
7.3
Step 38-Bromo-6-iodo-[1,2,4]-triazolo[1,5-a]pyridine Procedure:To a stirred solution of (E)-N-(3-bromo-5-iodopyridin-2-yl)-N-hydroxyformimidamide (4.2 g, 12.2 mmol) in THF (120 mL) at 0° C. was added dropwise 2,2,2-trifluoroacetic anhydride (10 mmol) slowly. After the addition, the reaction mixture was stirred at room temperature for 24 h. Then the reaction mixture was treated by saturated sodium bicarbonate solution until it reached pH=8. The solvent was evaporated at 40° C. at reduced pressure to give a crude product. This was purified by column chromatography (silica gel, 200-300 mesh, dichloromethane and methanol 100:1.5, v/v) to give 8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine (2.45 g, 62%) as a pale yellow solid. 1H NMR (300 MHz, CD3OD): δ 9.16 (d, 1H, J=1.2 Hz), 8.38 (s, 1H), 8.20 (d, 1H, J=0.9 Hz). LC/MS: 323.8 [M+H]+.
References:
US2012/309746,2012,A1 Location in patent:Page/Page column 33

1415728-06-1
0 suppliers
inquiry
![8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine](/CAS/20180703/GIF/1415314-10-1.gif)
1415314-10-1
8 suppliers
inquiry
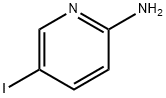
20511-12-0
460 suppliers
$6.00/1g
![8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine](/CAS/20180703/GIF/1415314-10-1.gif)
1415314-10-1
8 suppliers
inquiry
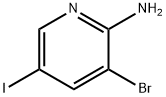
697300-73-5
136 suppliers
$33.00/250mg
![8-bromo-6-iodo-[1,2,4]triazolo[1,5-a]pyridine](/CAS/20180703/GIF/1415314-10-1.gif)
1415314-10-1
8 suppliers
inquiry