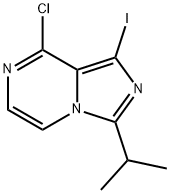
8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine synthesis
- Product Name:8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine
- CAS Number:1320266-92-9
- Molecular formula:C9H9ClIN3
- Molecular Weight:321.55
![8-chloro-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-90-7.gif)
1320266-90-7
![8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-92-9.gif)
1320266-92-9
General procedure for the synthesis of 8-chloro-1-iodo-3-isopropylimidazo[1,5-a]pyrazines from 8-chloro-3-isopropylimidazo[1,5-a]pyrazines: intermediate J (33 mg, 0.17 mmol) was dissolved in 0.18 mL of DMF and then added to a solution of N-iodosuccinimide (NIS, 39 mg, 0.17 mmol) dissolved in 0.6 mL of DMF. 0.17 mmol) solution. The reaction mixture was heated to 60 °C and the reaction was stirred at this temperature for 3 hours. After completion of the reaction, the mixture was cooled to room temperature. Subsequently, the reaction mixture was partitioned between 1 M Na2SO3 aqueous solution and dichloromethane. The aqueous layer was extracted three times (3X) with dichloromethane. All organic layers were combined, dried with anhydrous Na2SO4, filtered and concentrated in vacuum to give 34 mg (62% yield) of pure intermediate K. The product was characterized by 1H-NMR (300 MHz, CDCl3): δ 1.41 (d, 6H, J=6.9 Hz), 3.20-3.31 (m, 1H), 7.28 (d, 1H, J=5.1 Hz), and 7.65 (d, 1H, J=5.1Hz). The mass spectrum (ESI) showed (M+H)+ m/z of 322.1.
![8-chloro-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-90-7.gif)
1320266-90-7
19 suppliers
inquiry
![8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-92-9.gif)
1320266-92-9
22 suppliers
inquiry
Yield: 62%
Reaction Conditions:
with N-iodo-succinimide in N,N-dimethyl-formamide at 60; for 3 h;
Steps:
65
Intermediate J (33 mg, 0.17 mmol) dissolved in 0.18 mL of DMF was added to NIS (39 mg, 0.17 mmol) dissolved in 0.6 mL of DMF. The reaction mixture was heated to 60 °C for 3 h and then cooled to room temperature. The reaction mixture was partitioned between 1 M a2S03 and dichloromethane. The aqueous layer was then extracted with dichloromethane (3X). The organic layers were combined, dried over a2S04 and concentrated in vacuo to afford 34 mg (62% yield) of pure Intermediate K. .H-NMR (300 MHz, CDC13) δ 1.41 (d, 6H, / = 6.9 Hz), 3.20-3.31 (m, 1H), 7.28 (d, 1H, J= 5.1 Hz), 7.65 (d, 1H, J = 5.1 Hz). MS (ESI) (M+H)+ 322.1.
References:
UNIVERSITY OF WASHINGTON;VAN VOORHIS, Wesley, C.;HOL, Wilhelmus, G.J.;LARSON, Eric, T.;MALY, Dustin, James;MERRITT, Ethan;OJO, Kayode, K. WO2011/94628, 2011, A1 Location in patent:Page/Page column 107
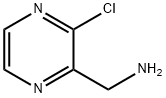
771581-15-8
114 suppliers
$105.00/50mg
![8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-92-9.gif)
1320266-92-9
22 suppliers
inquiry
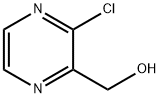
89283-32-9
86 suppliers
$26.00/100mg
![8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-92-9.gif)
1320266-92-9
22 suppliers
inquiry
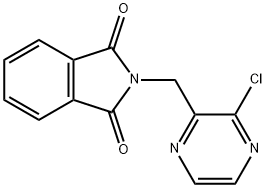
867165-55-7
12 suppliers
inquiry
![8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-92-9.gif)
1320266-92-9
22 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![8-chloro-1-iodo-3-isopropyliMidazo[1,5-a]pyrazine](/CAS/20150408/GIF/1320266-92-9.gif)
1320266-92-9
22 suppliers
inquiry