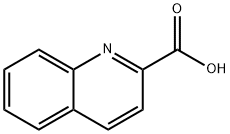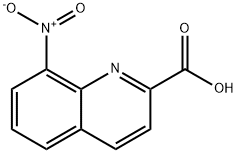
8-nitroquinoline-2-carboxylic acid synthesis
- Product Name:8-nitroquinoline-2-carboxylic acid
- CAS Number:15733-85-4
- Molecular formula:C10H6N2O4
- Molecular Weight:218.17
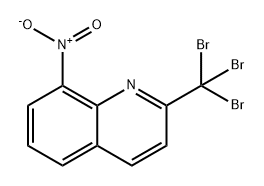
73257-75-7
0 suppliers
inquiry

15733-85-4
12 suppliers
inquiry
Yield:15733-85-4 97.2%
Reaction Conditions:
with sulfuric acid in lithium hydroxide monohydrate; for 24 h;Reflux;Concentration;
Steps:
Synthesis of 8-nitroquinoline-2-carboxylic acid
The synthesis was carried out with sulfuric acid of different concentrations indicated in Table 1. 2-Tribromomethyl-8-nitroquinoline (25.5 g, 60 mmol) was suspended in aqueous sulfuric acid (485 mL). The reaction mixture was refluxed with stirring until complete dissolution of 4, cooled to 80 °C, and poured into water (500 mL). The reaction mixture was hot filtered from the side products and poured into water (800 mL). After 10-12 h (at ~20 °C), a precipitate formed was filtered, washed with cold water (20 mL) on the filter. An analytical sample was obtained by recrystallization from isopropyl alcohol. The yields of compound 1 are given in Table 1. Melting point of compound 1 was 176-177 °C. Found (%): C, 55.02; H, 3.03; N, 12.71. C10H6N2O4. Calculated (%): C, 55.05; H, 2.77; N, 12.84; O, 29.33. 1H NMR, δ: 7.90 (dd, 1 H, C(6)H, J= 8.5 Hz, J= 9.0 Hz); 8.27 (d, 1 H, C(3)H, J= 9.0 Hz); 8.37(d, 1 H, C(5)H, J= 8.5 Hz); 8.40 (d, 1 H, C(4)H, J= 9.0 Hz); 8.77 (d, 1 H, C(7)H, J= 9.0 Hz); 13.75 (br.s, 1 H, -COOH). MS, m/z (I/Imax(%)): 218 [M] (20), 174 (100), 157 (18), 127 (40), 116 (45), 101 (30).
References:
Gadomsky, S. Ya.;Yakuschenko [Russian Chemical Bulletin,2016,vol. 65,# 3,p. 816 - 818][Izv. Akad. Nauk, Ser. Khim.,2016,# 3,p. 816 - 818,3]
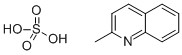
655-76-5
18 suppliers
inquiry

15733-85-4
12 suppliers
inquiry
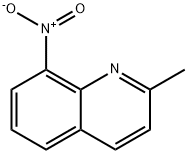
881-07-2
128 suppliers
$20.00/1g

15733-85-4
12 suppliers
inquiry
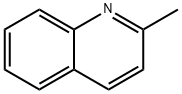
91-63-4
273 suppliers
$23.00/25g

15733-85-4
12 suppliers
inquiry