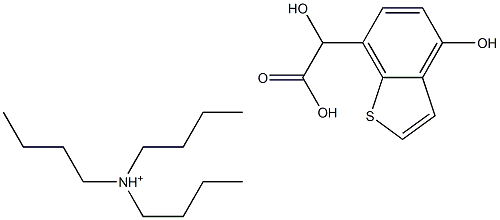
2-hydroxy-2-(4-hydroxy-1-benzothiophen-7-yl)acetate:tributylazanium synthesis
- Product Name:2-hydroxy-2-(4-hydroxy-1-benzothiophen-7-yl)acetate:tributylazanium
- CAS Number:817586-35-9
- Molecular formula:C22H36NO4S+
- Molecular Weight:410.5905
![benzo[b]thiophene-4-ol](/CAS/GIF/3610-02-4.gif)
3610-02-4
73 suppliers
$205.00/250mg
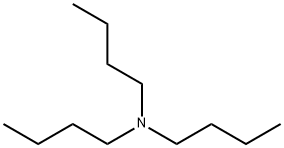
102-82-9
0 suppliers
$10.00/1ml
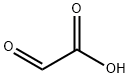
298-12-4
475 suppliers
$5.00/25g

817586-35-9
9 suppliers
inquiry
Yield:817586-35-9 53.1%
Reaction Conditions:
Stage #1: 4-hydroxybenzothiophene;Glyoxilic acidwith potassium hydroxide in water at 0 - 5; pH=11.5; for 3.5 h;
Stage #2: with hydrogenchloride in tert-butyl methyl ether;water; pH=~ 2 - ~ 7;
Stage #3: tributyl-amine in tert-butyl methyl ether;acetonitrile at 20 - 30;
Steps:
23 Tributylammonium Hydroxy-(4-hydroxy-benzo [b]thiophen-7-yl)-acetate
Example 23 Tributylammonium Hydroxy-(4-hydroxy-benzo [b]thiophen-7-yl)-acetate A 2 L reactor equipped with a mechanical stirrer, a thermometer, a dropping funnel, a sensor connected to a pH-meter and an argon inlet was charged under argon with 76.2 g (500 mmol) of 4-Hydroxybenzothiophene and 617.1 g (1100 mmol) of a 10% KOH aqueous solution. To the dark solution were added at 0-5° C. within 30 min ca. 85.91 g (580 mmol) of a 50% glyoxylic acid solution in water. If necessary, more glyoxylic acid is added such that the pH of the solution at the end of the addition was 11.5. After stirring for 3 h at 0-5° C., 200 ml of tert-butyl methyl ether were added to the reaction mixture followed by ca. 70 ml of 25% HCl solution in water such that the pH was ca. 7.0. The biphasic mixture was filtered through Speedex, then ca. 70 ml of 25% HCl solution in water were added to the aqueous phase such that the pH was ca. 2.0. After addition of 450 ml of tert-butyl methyl ether the organic phase was separated at room temperature and the aqueous phase washed with tert-butyl methyl ether. The combined organic phases were concentrated to a volume of ca. 300 ml and the residue was diluted with 50 ml of tert-butyl methyl ether and 100 ml of acetonitrile. To the resulting clear solution was added portionswise at 20-30° C. within 1 h a solution of 93.6 g (500 mmol) of tributylamine in 100 ml of tert-butyl methyl ether under seeding with crystals of the product. The resulting suspension was stirred over night at 20-30° C. and then filtered off. The filter cake was washed with 160 ml of tert-butyl methyl ether/acetonitrile 3:1 and the crystals dried over night at 60° C./10 mbar to afford 108.9 g (53.1%) of tributylammonium hydroxy-(4-hydroxy-benzo[b]thiophen-7-yl)-acetate as white crystals with a m.p. of ca. 200° C. (dec.).
References:
US2005/70714,2005,A1 Location in patent:Page/Page column 13