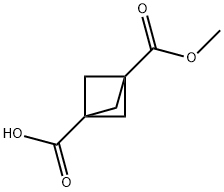
Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester synthesis
- Product Name:Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester
- CAS Number:83249-10-9
- Molecular formula:C8H10O4
- Molecular Weight:170.16
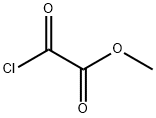
5781-53-3
![Tricyclo[1.1.1.01,3]pentane](/CAS/GIF/35634-10-7.gif)
35634-10-7
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
The general procedure for the synthesis of methyl bicyclo[1.1.1]pentane-1-carboxylate-3-carboxylate from oxalyl chloride monomethyl ester and the compound (CAS:35634-10-7) was as follows: first, a solution of [1.1.1]propeller in Et2O (500 mL; 161 mmol, 1 eq.) was prepared independently, along with ethyl chloroacetate (16.3 mL, 177 mmol, 1.1 eq.) solution in Et2O (49.7 mL). The entire reactor was flushed for 5 min using pure Et2O (pump 1: 4.0 mL/min, pump 2: 0.5 mL/min). Subsequently, a solution of reagent (2) was injected at a flow rate of 4.0 mL/min at 0 to -80 °C, followed by a solution of reagent (3) at a flow rate of 0.5 mL/min at room temperature. The photoreactor was placed in an ice bath (volume 58 mL; FEP tube rotated over a lamp). After the reaction mixture left the photoreactor, it was quenched using an alkaline aqueous solution of NaOH, KOH, NaHCO3, or KHCO3. After vigorous stirring for 2 h, the aqueous layer was separated and acidified to pH = 1. Subsequently, the mixture was extracted with DCM (3 × 500 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to afford 3-(methoxycarbonyl)bicyclo[1.1.1]pentane-1-carboxylic acid (9, 17.5 g, 103 mmol, 63% yield), the product being a pale yellow solid.

5781-53-3
246 suppliers
$8.00/10g
![Tricyclo[1.1.1.01,3]pentane](/CAS/GIF/35634-10-7.gif)
35634-10-7
15 suppliers
inquiry
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg
Yield:83249-10-9 63%
Reaction Conditions:
Stage #1:monomethyl oxalyl chloride;[1.1.1]propellane in diethyl ether at -80 - 0;
Stage #2: with water for 2 h;Alkaline conditions;
Steps:
3.a (a) 3-(methoxycarbonyl)bicyclo[l.l.l]pentane-l-carboxylic acid (9) using flow system
A solution of [l.l.ljpropellane in Et20 2 (500 mL; 161 mmol, 1 equiv.), and a solution of methyl chlorooxoacetate 3 (16.3 mL, 177 mmol, 1.1 equiv.) in Et20 (49.7 mL) were prepared independently. The entire reactor was flushed with pure Et20 (pump 1: 4.0 mL-min-1, pump 2: 0.5 mL-min-1) for 5 min. The solution of reagent (2) at 0 to -80 °C was then injected at a flow rate of 4.0 mL-min"1 and the solution of reagent (3) at room temperature was then injected at a flow rate of 0.5 mL-min-1 to the photoreactor (volume 58 mL; FEP tube was rotated on the lamp) in an ice bath. The reaction mixture was quenched with aqueous basic solution of NaOH, KOH, NaHC03 or KHC03, etc after the solution exits the photoreactor. After 2 h of vigorous stirring, the aqueous layer was separated and acidified until pH = 1. The mixture was then extracted with DCM (3 x 500 mL). The combined organic layers were dried over MgS04, filtered and concentrated under reduced pressure to provide 3- (methoxycarbonyl)bicyclo[l.l.l]pentane-l-carboxylic acid (9, 17.5 g, 103 mmol, 63%) as a pale yellow solid
References:
SPIROCHEM AG;SUZUKI, Yoshikazu;JIMENEZ-TEJA, Daniel;SALOMÉ, Christophe;FESSARD, Thomas WO2017/157932, 2017, A1 Location in patent:Page/Page column 27
![DiMethyl bicyclo[1.1.1]pentane-1,3-dicarboxylate](/CAS/20150408/GIF/115913-32-1.gif)
115913-32-1
103 suppliers
$26.00/100mg
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg
![Bicyclo[1.1.1]pentane-1-carboxylic acid, 3-(chlorocarbonyl)-, methyl ester](/CAS/20210111/GIF/110371-22-7.gif)
110371-22-7
4 suppliers
inquiry
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg
![Methyl-3-phenylbicyclo[1.1.1]pentane-1-carboxylate](/CAS/20180629/GIF/83249-09-6.gif)
83249-09-6
18 suppliers
inquiry
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid](/CAS/GIF/56842-95-6.gif)
56842-95-6
128 suppliers
$38.00/100mg
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg