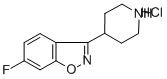
6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride synthesis
- Product Name:6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride
- CAS Number:84163-13-3
- Molecular formula:C12H14ClFN2O
- Molecular Weight:256.7
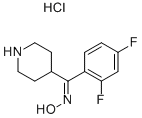
135634-18-3

84163-13-3
The general procedure for the synthesis of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride from (2,4-difluorophenyl)-(4-piperidinyl)methanone oxime hydrochloride was as follows: potassium hydroxide (27 g) was dissolved in 600 mL of methanol, followed by addition of (2,4-difluorophenyl)-(4-piperidinyl)methanone oxime hydrochloride (55 g). The reaction mixture was heated to reflux for about 2.5 hours. After completion of the reaction, it was cooled to room temperature, dried by adding an appropriate amount of anhydrous magnesium sulfate and stirred for about 1 hour. The filtrate was concentrated under reduced pressure to remove the solvent. To the concentrate was added 500mL of acetone, stirred at room temperature for about 0.5 hours, and filtered to remove insoluble material. Hydrochloric acid was slowly added to the filtrate and the pH was adjusted to 2?3, precipitating a white solid. The solid was collected by filtration and dried to give 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (35 g) with 0.4% methanol.
84163-46-2
7 suppliers
inquiry

84163-13-3
370 suppliers
$10.00/1g
Yield:84163-13-3 97.8%
Reaction Conditions:
with N-methylcyclohexylamine;potassium tert-butylate in water;toluene for 6 h;Reflux;
Steps:
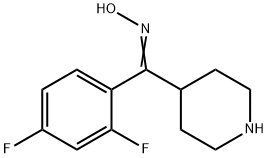
5; 6; 7; 8 Example 8Preparation of 6-fluoro-3-(4-piperidinyl)-1,2-benzisazole hydrochloride (IV).
In a 1000 mL sealed four-mouth reaction bottle,Add 48.1 g of 2,4-difluorophenyl-4-piperidinone oxime (III),Potassium tert-butoxide 44.9 g,250 mL of water and 250 mL of toluene,Stir and heat to reflux for 6 h.The reaction solution is cooled to 5 to 10 ° C,Liquid separation,The aqueous layer was extracted with 250 mL of toluene.The toluene phases were combined in a 1000 mL four-necked flask.Slowly add hydrochloric acid to adjust the pH to 2~3.A large amount of white solid precipitated,Stop stirring,Filtered white solid6-fluoro-3-(4-piperidinyl)-1,2-benzisazole hydrochloride (IV) 50.2 g, yield 97.8%.
References:
Zhejiang University Of Technology Shangyu Institute Co., Ltd.;Fang Biao;Ke Junliang;Xu Meng CN109438443, 2019, A Location in patent:Paragraph 0030-0037

135634-18-3
150 suppliers
$43.00/1g

84163-13-3
370 suppliers
$10.00/1g
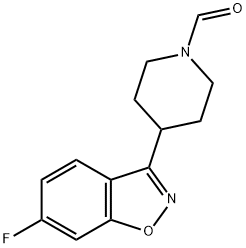
84163-41-7
1 suppliers
inquiry

84163-13-3
370 suppliers
$10.00/1g
![1-Piperidinecarboxaldehyde, 4-[(2,4-difluorophenyl)(hydroxyimino)methyl]-](/CAS/20210305/GIF/84162-94-7.gif)
84162-94-7
1 suppliers
inquiry

84163-13-3
370 suppliers
$10.00/1g
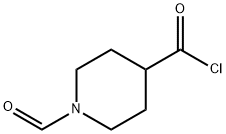
84163-43-9
6 suppliers
inquiry

84163-13-3
370 suppliers
$10.00/1g