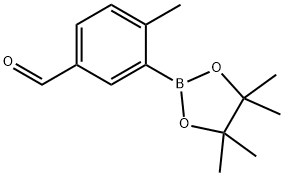
4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde synthesis
- Product Name:4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde
- CAS Number:847560-50-3
- Molecular formula:C14H19BO3
- Molecular Weight:246.11
36276-24-1

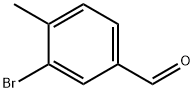
73183-34-3

847560-50-3
General procedure for the synthesis of 4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde from 3-bromo-4-methylbenzaldehyde and pinacol bis(borate) ester: To a stirred solution of 3-bromo-4-methylbenzaldehyde (3.0 g, 15.1 mmol) in 1,4-dioxane (30 mL) was added pinacol bis(borate) ester ( 4.5 g, 17.7 mmol) and potassium acetate (2.98 g, 30.4 mmol). The reaction mixture was degassed with nitrogen for 20 min. Subsequently, Pd(dppf)Cl2 (0.62 g, 0.75 mmol) was added. The reaction system was heated and reacted at 90 °C overnight. After completion of the reaction, the mixture was diluted with water and extracted with ethyl acetate. The organic layer was washed sequentially with water and saturated saline and then concentrated. Purification by combiflash column chromatography afforded the target product 4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde (3.4 g, 91.39% yield) as a yellow semi-solid.LCMS: (M + H)+ = 247.2; 1H NMR (CDCl3, 300MHz) δ 9.99 (s , 1H), 8.26 (s, 1H), 7.82-7.85 (dd, J=7.8, 1.5 Hz, 1H), 7.26-7.33 (m, 1H), 2.62 (s, 3H), 1.37 (s, 12H).

36276-24-1
128 suppliers
$35.00/1g

73183-34-3
587 suppliers
$6.00/5g

847560-50-3
40 suppliers
$26.00/100mg
Yield:847560-50-3 91.39%
Reaction Conditions:
Stage #1: 3-bromo-4-methyl-benzaldehyde;bis(pinacol)diboranewith potassium acetate in 1,4-dioxane; for 0.333333 h;Inert atmosphere;
Stage #2: with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 90;
Steps:
Intermediate 5a: 4-Methyl-3-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl )benzal dehyd e
To a stirred solution of intermediate 4a (3.0 g, 15.1 mmol) in 1,4 dioxone (30 mL), bis(pinocolate)diboran (4.5 g, 17.7 mmol) and potassium acetate (2.98 g, 30.4 mmol) was added. The resultant was degasified with nitrogen for 20 mm. Then, Pd(dppf)C12 (0.62 g, 0.75 mmol) was added to it. It was heated at 90°C over night. The reaction mixture was diluted with water and extracted with ethyl acetate; the organic layer waswashed with water and brine solution and was then concentrated. The product was purified by combiflash to yield the title product (3.4 g, 91 .39%) as a yellow semi solid.LCMS: (M+H) =247.2; 1H NMR: (ODd3, 300MHz) 69.99(s, 1H), 8.26 (5, 1H), 7.82- 7.85 (dd, 1H), 7.26-7.33 (m, 1H), 2.62 (5, 3H), 1.37 (5, 12H).
References:
WO2014/202580,2014,A1 Location in patent:Page/Page column 79
104-87-0
516 suppliers
$6.00/25g

847560-50-3
40 suppliers
$26.00/100mg