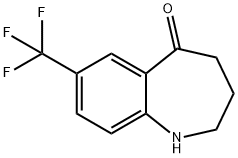
5H-1-Benzazepin-5-one, 1,2,3,4-tetrahydro-7-(trifluoroMethyl)- synthesis
- Product Name:5H-1-Benzazepin-5-one, 1,2,3,4-tetrahydro-7-(trifluoroMethyl)-
- CAS Number:851045-54-0
- Molecular formula:C11H10F3NO
- Molecular Weight:229.2
amino]-5-(trifluoromethyl)-, methyl ester](/CAS/20210305/GIF/851045-53-9.gif)
851045-53-9
0 suppliers
inquiry

851045-54-0
9 suppliers
inquiry
Yield:851045-54-0 45%
Reaction Conditions:
Stage #1: 2-[tert-butoxycarbonyl-(3-methoxycarbonyl-propyl)-amino]-5-trifluoromethyl-benzoic acid methyl esterwith potassium tert-butylate in toluene at 70; for 2.5 h;
Stage #2: with water;acetic acid in dichloromethane;toluene at 20;
Stage #3: with hydrogenchloride;water in acetic acid at 100; for 4 h;
Steps:
139.4
Step 4. Preparation of 7-Trifluoromethyl-1, 2,3, 4-tetrahydro-benzo [b] azepin-5-one. Add a solution of methyl 2- [tert-Butoxycarbonyl- (3-methoxycarbonyl-propyl)- amino] -5-trifluoromethyl-benzoic acid methyl ester (0.5 g, 1.3 mmol) in toluene (25 mL) to a suspension of potassium tert-butoxide (0.3 g, 2.6 mmol) in toluene (75 mL) at 70 °C, under an atmosphere of nitrogen, over a period of 30 min. After 2h, cool the reaction to room temperature and quench the reaction with acetic acid (2.6 mmol). Dilute the reaction with water (100 mL) and dichloromethane (100 mL). Separate the organic phase, dry over sodium sulfate, and filter. Remove the solvent under vacuum then dissolve the crude intermediate in acetic acid (20 mL). Dilute with conc. HCl (20 mL) and water (10 mL) and heat the mixture to 100 °C for 4 h. After cooling to room temperature neutralize the reaction with 5N NaOH while cooling in an ice bath. Extract the organics with ethyl acetate (3xlOOmL), dry over sodium sulfate, and filter. Remove the solvent under vacuum and chromatograph the product over silica gel using ethyl acetate/hexane (5- 20%) to elute. This provides the title compound as an off white solid (0.13 g, 45%): H NMR (CDC13, 400 MHz) 8 2. 22-2. 29 (m, 2H), 2.88 (t, J = 6.4 Hz), 3.35 (t, J = 6.8 Hz), 4.96 (bs, 1H), 6.84 (d, J = 8.8 Hz, 1H), 7.46 (dd, J = 2.0, 8.8 Hz, 1H), 8.02 (s, 1H).
References:
WO2005/37796,2005,A1 Location in patent:Page/Page column 139-140