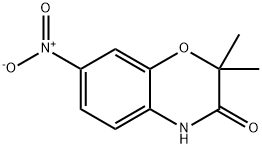
2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE synthesis
- Product Name:2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE
- CAS Number:85160-83-4
- Molecular formula:C10H10N2O4
- Molecular Weight:222.2
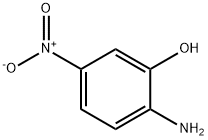
600-00-0
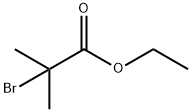
121-88-0
![2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/85160-83-4.gif)
85160-83-4
1. 125 mM potassium carbonate was added to 100 mL of anhydrous N,N-dimethylformamide (DMF). 2. 50 mM 2-amino-5-nitrophenol was added to the above solution and stirred at room temperature for 1 hr until completely dissolved. 3. A DMF solution containing 50 mM ethyl 2-bromo-2-methylpropionate was added dropwise slowly in an ice bath. 4. After completion of the addition, the reaction mixture was kept in an ice bath with continued stirring for half an hour. 5. The reaction mixture was warmed slowly to room temperature and then heated to 90 °C for 12 hr. 6. After completion of the reaction, the reaction mixture was cooled and some solvent was removed by decompression distillation. 7. After the dropwise addition, the reaction mixture was kept in an ice bath with stirring for half an hour. 5. The reaction mixture was slowly warmed to room temperature and then heated to 90 °C for 12 hours. 6. After completion of the reaction, the reaction mixture was cooled and some of the solvent was removed by distillation under reduced pressure. 7. 300-500 mL of ice water was slowly added to the remaining solution with stirring to induce the precipitation of solids. 8. The mixture was kept in an ice bath with stirring for half an hour and then filtered to collect the solids. 9. The filter cake was vacuumed. 10. The solid was collected by filtration.9. The filter cake was dried under vacuum to give 8.32 g of the intermediate in brown powder form in 75.0% yield.10. The intermediate can be used directly in the subsequent reaction without further purification.

600-00-0
264 suppliers
$13.00/5g

121-88-0
32 suppliers
$10.00/5g
![2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/85160-83-4.gif)
85160-83-4
45 suppliers
$27.00/100mg
Yield:85160-83-4 348.6 g
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 26 - 30; for 24 h;
Steps:
1.1 (1)
Preparation of 2,2-dimethyl-7-nitro-2H-1,4-benzoxazin-3(4H)-one
(1)
Preparation of 2,2-dimethyl-7-nitro-2H-1,4-benzoxazin-3(4H)-one
To a solution of 2-amino-5-nitrophenol (200 g) in dimethylsulfoxide (1000 mL) was added anhydrous potassium carbonate (269 g) under stirring at 30°C or lower, then to the mixture was added ethyl 2-bromo-2-methylpropionate (278.4 g) at 30°C or lower, and the mixture was stirred at 26°C for 24 hours.
To the reaction mixture was added water (2000 mL) at 40°C or lower, and then stirred at room temperature for 3 hours.
The reaction product was collected by filtration, and successively washed with dimethylsulfoxide/water (2:1, 800 mL) and water (3200 mL) to obtain the title compound (348.6 g) as a yellow solid (wet material) (yield: 121%, purity: 96%).
MS: ESI-MS m/Z: 223 [M+H]+
1 H-NMR (CDCl3): δ 1.59 (6H, s), 6.95 (1H, d), 7.87 (1H, d), 7.92 (1H, dd), 9.38 (1H, brs)
References:
EP2883870,2015,A1 Location in patent:Paragraph 0073; 0074

600-00-0
264 suppliers
$13.00/5g
![2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/85160-83-4.gif)
85160-83-4
45 suppliers
$27.00/100mg
![Propionitrile, 2,2'-[[2-(1-cyano-1-methylethoxy)-4-nitrophenylimino]oxy]bis[2-methyl- (6CI)](/CAS/20240320/GIF/114254-56-7.gif)
114254-56-7
0 suppliers
inquiry
![2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/85160-83-4.gif)
85160-83-4
45 suppliers
$27.00/100mg

121-88-0
32 suppliers
$10.00/5g
![2,2-DIMETHYL-7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/85160-83-4.gif)
85160-83-4
45 suppliers
$27.00/100mg