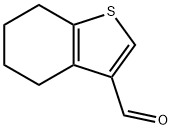
4,5,6,7-tetrahydro-1-benzothiophene-3-carbaldehyde(SALTDATA: FREE) synthesis
- Product Name:4,5,6,7-tetrahydro-1-benzothiophene-3-carbaldehyde(SALTDATA: FREE)
- CAS Number:851634-60-1
- Molecular formula:C9H10OS
- Molecular Weight:166.24
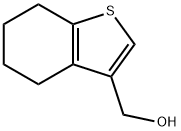
851634-59-8
5 suppliers
inquiry

851634-60-1
18 suppliers
$45.00/50mg
Yield:851634-60-1 88%
Reaction Conditions:
Stage #1: (4,5,6,7-tetrahydrobenzo[b]thiophene-3-yl)methanolwith Dess-Martin periodane in dichloromethane at 20; for 0.333333 h;
Stage #2: with sodium hydroxide in diethyl ether;dichloromethane;water; for 0.25 h;
Steps:
28.2
;A solution of 4,5,6,7-tetrahydro-1-benzothien-3-ylmethanol (Step 1, 320 mg, 1.9 mmol) in dichloromethane (10 mL) was added to a stirred solution of 1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one (0.92 g, 2.1 mmol) in dichloromethane (10 mL) under nitrogen and stirred at room temperature for 20 minutes. Diethyl ether (50 mL) and 1N sodium hydroxide solution (25 mL) were added and the mixture stirred vigorously for 15 minutes. The organic layer was washed with sodium hydroxide, water and brine, dried over MgSO4 and evaporated in vacuo to give 4,5,6,7-tetrahydro-1-benzothiophene-3-carbaldehyde (280 mg, 88%). 1H NMR (360 MHz, CDCl3) δ 9.88 (1H, s), 7.85 (1H, s), 2.92-2.88 (2H, m), 2.76 (2H, t, J=6.0 Hz), 1.88-1.76 (4H, m).
References:
US2005/101586,2005,A1 Location in patent:Page/Page column 13
14559-12-7
22 suppliers
$180.00/500mg

851634-60-1
18 suppliers
$45.00/50mg
![ETHYL 2-AMINO-4,5,6,7-TETRAHYDROBENZO[B]THIOPHENE-3-CARBOXYLATE](/CAS/GIF/4506-71-2.gif)
4506-71-2
200 suppliers
$6.00/1g

851634-60-1
18 suppliers
$45.00/50mg