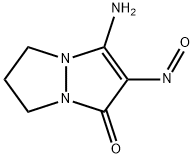
3-amino-2-nitroso-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one synthesis
- Product Name:3-amino-2-nitroso-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one
- CAS Number:857035-96-2
- Molecular formula:C6H8N4O2
- Molecular Weight:168.15
![3-AMino-6,7-dihydropyrazolo[1,2-a]pyrazol-1(5H)-one hydrochloride](/CAS/20150408/GIF/358360-19-7.gif)
358360-19-7
30 suppliers
inquiry
![3-amino-2-nitroso-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one](/CAS/20180529/GIF/857035-96-2.gif)
857035-96-2
6 suppliers
inquiry
Yield:857035-96-2 85%
Reaction Conditions:
Stage #1: 3-amino-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one hydrochloridewith hydrogenchloride in water at 20;
Stage #2: with sodium nitrite in water at 0 - 5; for 1.5 h;
Stage #3: with sodium hydroxide in water at 0 - 5; pH=8;
Steps:
5 Synthesis of 3-amino-2-nitroso-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one: 2
43 g (0.245 mol) of 3-amino-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one hydrochloride were dissolved, with stirring, at room temperature, in a mixture of 180 ml of water and 35 ml of 35% hydrochloric acid in a 500 ml three-necked flask. The mixture was cooled to 0° C. and a solution of 17.3 g of sodium nitrite (0.25 mol) in 20 ml of water was added dropwise over 30 minutes. The temperature of the reaction medium was maintained at a range of from 0 to +5° C. throughout the addition and for one hour after the end of the addition. The reaction medium was brought to pH 8 by adding sodium hydroxide, with stirring, while maintaining the temperature at a range of from 0 to 5° C. The 3-amino-2-nitroso-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one 2 precipitates in the form of a red-orange solid, which was filtered off on a No. 4 sinter funnel, slurried in a minimum amount of 2-propanol, washed with diisopropyl ether and dried under vacuum in the presence of phosphorus pentoxide. 35 g of orange-red product were thus obtained (yield: 85%). The NMR (1H 400 MHz and 13C 100.61 MHz DMSO d6) and mass spectra were in accordance with the expected structure 2.
References:
US2006/277693,2006,A1 Location in patent:Page/Page column 22-23