
2-(1,2-dichloro-1,2,2-trifluoroethoxy)-1,1,1,2,3,3,3-heptafluoropropane synthesis
- Product Name:2-(1,2-dichloro-1,2,2-trifluoroethoxy)-1,1,1,2,3,3,3-heptafluoropropane
- CAS Number:85720-81-6
- Molecular formula:C5Cl2F10O
- Molecular Weight:336.9429
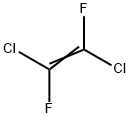
598-88-9
71 suppliers
inquiry
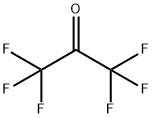
684-16-2
0 suppliers
$120.00/5g

85720-81-6
4 suppliers
inquiry
76-14-2
0 suppliers
$125.00/25g

375-45-1
47 suppliers
$74.00/5g
Yield:-
Reaction Conditions:
with fluorine at -80; for 4 h;
Steps:
8 EXAMPLE 8; Synthesis of (CF3)2-CF-O-CFCl-CF2Cl
Into the equipment of the Example 1, maintained at a temperature of -80° C., 16.8 g of CF3C(O)CF3 are loaded. 1.7 Nl/h of fluorine diluited with nitrogen (molar ratio fluorine/nitrogen 1/2.5) and 1.5 Nl/h di CFCl=CFCl are then fed. The reaction is continued for 4 hours and at the end the reactor is discharged.The reaction mixture is analyzed by gaschromatography. It is found that both the conversions of CFC 1112 and of CF3C(O)CF3 are 100%. The reaction mixture is distilled. 19.5 g of a compound having formula (CF3)2-CF-O-CFCl-CF2Cl (19F NMR) are recovered. Selectivity with respect to CF3C(O)CF3 is 57.0% and with respect to CFC 1112 is of 20.9%. It is found that together with the synthesis of the above compound, also the fluorination raction of CFC 1112 to give CFC 114 and of fluorodimerization to CF2Cl-CFCl-CFCl-,CF2Cl took place in the reactor. Selectivity calculated with respect to CFC 1112, for the product CFC 114 is 62.7% and for the CFC 1112 dimer is 1.6%. The molar balance is 99% for CFC 1112 and 90% for CF3C(O)CF3.
References:
US2004/30146,2004,A1 Location in patent:Page 4