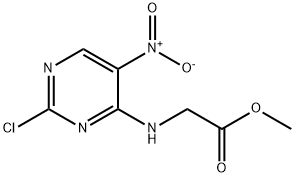
Methyl 2-(2-chloro-5-nitropyrimidin-4-ylamino)acetate synthesis
- Product Name:Methyl 2-(2-chloro-5-nitropyrimidin-4-ylamino)acetate
- CAS Number:859307-58-7
- Molecular formula:C7H7ClN4O4
- Molecular Weight:246.61
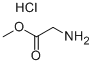
5680-79-5
502 suppliers
$3.66/25g
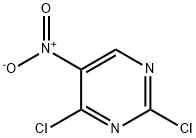
49845-33-2
374 suppliers
$5.00/1g

859307-58-7
11 suppliers
inquiry
Yield:859307-58-7 81.8%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20; for 8 h;
Steps:
1.1 Step 1 Synthesis of Intermediate 2
Dissolve 2,4-dichloro-5-nitropyrimidine (3.00g, 15.47mmol) in 60mL dichloromethane,After cooling to 0 in an ice bath, add glycine methyl ester hydrochloride (1.94g, 15.47mmol)And N,N-diisopropylethylamine (5.00g, 38.67mmol),Then the reaction temperature was raised to room temperature, and the reaction was continued for 8 hours.After the reaction is complete, pour the reaction liquid into the water,Extract with dichloromethane, wash the organic layer with saturated brine,Dry over Na2SO4 overnight.The desiccant was filtered off, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography.3.12 g of white solid was obtained, with a yield of 81.80%.
References:
CN112939979,2021,A Location in patent:Paragraph 0022; 0028; 0030-0031