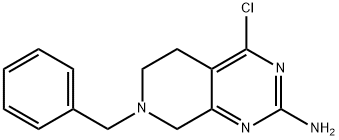
7-benzyl-4-chloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-amine synthesis
- Product Name:7-benzyl-4-chloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-amine
- CAS Number:859825-79-9
- Molecular formula:C14H15ClN4
- Molecular Weight:274.75
![2-AMino-7-benzyl-5,6,7,8-tetrahydro-3H-pyrido[3,4-d]pyriMidin-4-one](/CAS/20150408/GIF/62458-92-8.gif)
62458-92-8
13 suppliers
$200.00/1 G
![7-benzyl-4-chloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-amine](/CAS/GIF/859825-79-9.gif)
859825-79-9
24 suppliers
inquiry
Yield: 83%
Reaction Conditions:
Stage #1:2-amino-7-benzyl-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4(4aH)-one with N,N-dimethyl-aniline;trichlorophosphate in 1,2-dichloro-ethane at 90; for 0.75 h;
Stage #2: with sodium hydrogencarbonate in water
Steps:
17.B
B. 2-Benzyl-5-chloro-1, 2,3, 4-tetrahydroisoquinolin-7-amine [00264] ZV ethyllånilliïe : 4 g, 11. 4 mmol) and phosphorus oxychloride (14 g, 91. 2 mmol) were added to the solution of 2-amino-7-benzyl-5, 6, 7, 8-tetrahydropyrido [3, 4- d] pyfimidin-4 (4aH)-one (2.9 g, 11.4 mmol) in 1,2-dichloroethane (23 mL, anhydrous) and were stirred in a preheated oil bath at 90 °C for 45 min. The reaction mixture was poured into ice, neutralized by solid sodium carbonate, extracted by ethyl acetate, and the dark brown semi solid was dissolved in methanol. The combined ethyl acetate and methanol solution was dried over sodium sulfate. Solvent was removed in vacuo and product was obtained as a light brown solid (2.6 g, 83%).
References:
RENOVIS, INC. WO2005/66171, 2005, A1 Location in patent:Page/Page column 81-82
![2-AMino-7-benzyl-5,6,7,8-tetrahydro-3H-pyrido[3,4-d]pyriMidin-4-one](/CAS/20150408/GIF/62458-92-8.gif)
62458-92-8
13 suppliers
$200.00/1 G
![7-benzyl-4-chloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-amine](/CAS/GIF/859825-79-9.gif)
859825-79-9
24 suppliers
inquiry
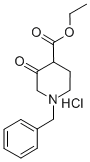
52763-21-0
242 suppliers
$6.00/1g
![7-benzyl-4-chloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-amine](/CAS/GIF/859825-79-9.gif)
859825-79-9
24 suppliers
inquiry