
1-(tert-butyl) 3-ethyl (3S,4R)-4-aminopiperidine-1,3-dicarboxylate synthesis
- Product Name:1-(tert-butyl) 3-ethyl (3S,4R)-4-aminopiperidine-1,3-dicarboxylate
- CAS Number:859855-31-5
- Molecular formula:C13H24N2O4
- Molecular Weight:272.34
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

859855-31-5
14 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethanol at 40; under 2585.81 Torr; for 24 h;
Steps:
75.3 Step 3: Synthesis of 1-(tert-butyl) 3-ethyl (3S,4R)-4-aminopiperidine-1,3-dicarboxylate
To a solution of 1-(tert-butyl) 3-ethyl (3S,4R)-4-(((R)-1-phenylethyl)amino)piperidine-1,3- dicarboxylate (39 g, 104 mmol, 1 equiv) in EtOH (156 mL) was added Pd/C (13 g, 10 % purity). The resulting mixture was stirred under H2 (50 psi) at 40 °C for 24 h then filtered through celite, washed with EtOAc (3 x 200 mL), and concentrated under reduced pressure to afford 1-(tert-butyl) 3-ethyl (3S,4R)-4- aminopiperidine-1,3-dicarboxylate (28 g, 99% yield) as a colorless liquid.1H NMR (400MHz, CDCl3) δ 4.14 - 4.02 (m, 2H), 3.70 - 3.52 (m, 3H), 3.44 - 3.25 (m, 3H), 1.76 - 1.56 (m, 2H), 1.45 - 1.33 (m, 9H), 1.27 - 1.14 (m, 3H).
References:
REVOLUTION MEDICINES, INC. WO2021/108683, 2021, A1 Location in patent:Page/Page column 230; 232
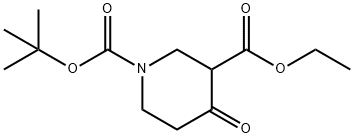
98977-34-5
203 suppliers
$5.00/250mg

859855-31-5
14 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

859855-31-5
14 suppliers
inquiry