
1-BENZYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:1-BENZYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER
- CAS Number:861135-90-2
- Molecular formula:C12H12N2O2
- Molecular Weight:216.24
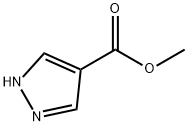
51105-90-9
113 suppliers
$9.00/100mg

100-39-0
429 suppliers
$10.00/10g

861135-90-2
4 suppliers
inquiry
Yield:861135-90-2 85%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 20; for 18 h;Inert atmosphere;
Steps:
1 Example 1: Preparation of Methyl 1 -Benzyl- lH-pyrazole-4-carboxylate (16)
To a 250 ml jacketed flask with stir-bar, thermocouple and positive nitrogen stream, were added methyl lH-pyrazole-4-carboxylate 15a (10 g, 77.7 mmol, 1.0 equiv.) and K2CO3(21.9 g, 158 mmol, 2.04 equiv.) followed by dimethylformamide (DMF) (80 ml). The mixture was cooled to 0 °C. Benzyl bromide (11.3 ml, 93.3 mmol, 1.2 equiv.) was charged over 10 minutes. The reaction mixture was brought to room temperature and stirred for 18 hours. Water (50 ml) was added and the mixture was transferred to a 500 ml separating funnel. The mixture was extracted twice with ethyl acetate (150 ml). The combined organic extract was washed with brine (30 ml), dried over Na2SC>4, and concentrated in vacuo to give a colorless residue, which crystallized upon standing to provide methyl 1 -benzyl- lH-pyrazole-4-carboxylate 16a (14. lg, 85% yield). 'H-NMR (400MHz, CDCh): d 7.85 (s, 1H), 7.75 (s, 1H), 7.31-7.22 (m, 3H), 7.18-7.13 (m, 2H), 5.21 (s, 2H), 3.71 (s, 3H).
References:
WO2022/6136,2022,A1 Location in patent:Paragraph 58-59