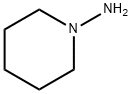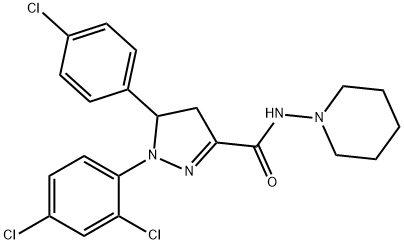
1H-Pyrazole-3-carboxaMide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-N-1-piperidinyl- synthesis
- Product Name:1H-Pyrazole-3-carboxaMide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-N-1-piperidinyl-
- CAS Number:861151-12-4
- Molecular formula:C21H21Cl3N4O
- Molecular Weight:451.78
Yield:861151-12-4 57%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 25;
Steps:
1.b
Under nitrogen atmosphere N-aminopiperidine (0.6 mL, 5.6 mmoles) and triethylamine (4 mL) were dissolved in methylene chloride (25 mL). The resulting mixture was ice-cooled down to 0°C and a solution of 5- (4-chlorophenyl)-1- (2, 4- dichlorophenyl)-4, 5-dihydro-pyrazole-3-carboxylic acid chloride obtained in step (c) in methylene chloride (15 mL) was added dropwise. The resulting reaction mixture was stirred at room temperature (approximately 25 °C) overnight. Afterwards the reaction mixture was washed with water, followed by a saturated aqueous solution of sodium bicarbonate, then again with water, dried over sodium sulfate, filtered and evaporated to dryness in a rotavapor. The resulting crude solid was crystallized from ethanol. The crystallized solid was removed via filtration and the mother liquors were concentrated to yield a second fraction of crystallized product. The two fractions were combined to give a total amount of 1.7 g (57% of theoretical yield) of N-piperidinyl-5- (4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4, 5-dihydropyrazole-3-carboxamide having a melting point of 183-186°C. IR (KBr, cm~1) : 3222,9, 2934,9, 1647,4, 1474,7, 1268,3, 815,6. 1H NMR (CDCI3, 8) : 1,4 (m, 2H), 1,7 (m, 4H), 2,8 (m, 4H), 3,3 (dd, J=6,1 y 18,3Hz, 1H), 3,7 (dd, J=12, 5 and 18, 3 Hz, 1H), 5,7 (dd, J=6,1 and 12,5 Hz, 1H), 7,0-7, 2 (m, 6H), 7,4 (s, 1 H).
References:
WO2005/77909,2005,A1 Location in patent:Page/Page column 49
![1H-Pyrazole-3-carboxamide, N-[2,6-bis(1H-benzotriazol-1-yl)-1-piperidinyl]-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-](/CAS/20210305/GIF/920526-52-9.gif)
920526-52-9
0 suppliers
inquiry

861151-12-4
3 suppliers
inquiry